Mechanistic studies on bleomycin-mediated DNA damage: multiple binding modes can result in double-stranded DNA cleavage
- PMID: 18492718
- PMCID: PMC2441780
- DOI: 10.1093/nar/gkn302
Mechanistic studies on bleomycin-mediated DNA damage: multiple binding modes can result in double-stranded DNA cleavage
Abstract
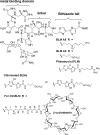
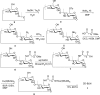
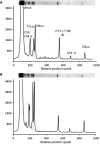
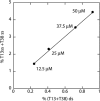
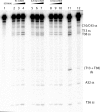
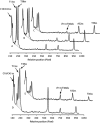
The bleomycins (BLMs) are a family of natural glycopeptides used clinically as antitumor agents. In the presence of required cofactors (Fe(2+) and O(2)), BLM causes both single-stranded (ss) and double-stranded (ds) DNA damage with the latter thought to be the major source of cytotoxicity. Previous biochemical and structural studies have demonstrated that BLM can mediate ss cleavage through multiple binding modes. However, our studies have suggested that ds cleavage occurs by partial intercalation of BLM's bithiazole tail 3' to the first cleavage site that facilitates its re-activation and re-organization to the second strand without dissociation from the DNA where the second cleavage event occurs. To test this model, a BLM A5 analog (CD-BLM) with beta-cyclodextrin attached to its terminal amine was synthesized. This attachment presumably precludes binding via intercalation. Cleavage studies measuring ss:ds ratios by two independent methods were carried out. Studies using [(32)P]-hairpin technology harboring a single ds cleavage site reveal a ss:ds ratio of 6.7 +/- 1.2:1 for CD-BLM and 3.4:1 and 3.1 +/- 0.3:1 for BLM A2 and A5, respectively. In contrast with BLM A5 and A2, however, CD-BLM mediates ds-DNA cleavage through cooperative binding of a second CD-BLM molecule to effect cleavage on the second strand. Studies using the supercoiled plasmid relaxation assay revealed a ss:ds ratio of 2.8:1 for CD-BLM in comparison with 7.3:1 and 5.8:1, for BLM A2 and A5, respectively. This result in conjunction with the hairpin results suggest that multiple binding modes of a single BLM can lead to ds-DNA cleavage and that ds cleavage can occur using one or two BLM molecules. The significance of the current study to understanding BLM's action in vivo is discussed.
Figures

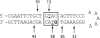

References
-
- Umezawa H, Maeda K, Takeuchi T, Okami Y. New antibiotics, bleomycin A and B. J. Antibiot. 1966;19:200–209. - PubMed
-
- Sikic BI, Rozencweig M, Carter SK. Orlando, Florida: Academic Press; 1985. Bleomycin Chemotherapy.
-
- Stubbe J, Kozarich JW. Mechanisms of bleomycin-induced DNA degradation. Chem. Rev. 1987;87:1107–1136.
-
- Burger RM. Cleavage of nucleic acids by bleomycin. Chem. Rev. 1998;98:1153–1169. - PubMed

