Transglutaminases: widespread cross-linking enzymes in plants
- PMID: 18492735
- PMCID: PMC2712369
- DOI: 10.1093/aob/mcn075
Transglutaminases: widespread cross-linking enzymes in plants
Abstract
Background: Transglutaminases have been studied in plants since 1987 in investigations aimed at interpreting some of the molecular mechanisms by which polyamines affect growth and differentiation. Transglutaminases are a widely distributed enzyme family catalysing a myriad of biological reactions in animals. In plants, the post-translational modification of proteins by polyamines forming inter- or intra-molecular cross-links has been the main transglutaminase reaction studied.
Characteristics of plant transglutaminases: The few plant transglutaminases sequenced so far have little sequence homology with the best-known animal enzymes, except for the catalytic triad; however, they share a possible structural homology. Proofs of their catalytic activity are: (a) their ability to produce glutamyl-polyamine derivatives; (b) their recognition by animal transglutaminase antibodies; and (c) biochemical features such as calcium-dependency, etc. However, many of their fundamental biochemical and physiological properties still remain elusive.
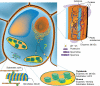
Transglutaminase activity is ubiquitous: It has been detected in algae and in angiosperms in different organs and sub-cellular compartments, chloroplasts being the best-studied organelles.
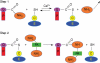
Possible roles: Possible roles concern the structural modification of specific protein substrates. In chloroplasts, transglutaminases appear to stabilize the photosynthetic complexes and Rubisco, being regulated by light and other factors, and possibly exerting a positive effect on photosynthesis and photo-protection. In the cytosol, they modify cytoskeletal proteins. Preliminary reports suggest an involvement in the cell wall construction/organization. Other roles appear to be related to fertilization, abiotic and biotic stresses, senescence and programmed cell death, including the hypersensitive reaction.
Conclusions: The widespread occurrence of transglutaminases activity in all organs and cell compartments studied suggests a relevance for their still incompletely defined physiological roles. At present, it is not possible to classify this enzyme family in plants owing to the scarcity of information on genes encoding them.
Figures

Similar articles
-
Plant Transglutaminases: New Insights in Biochemistry, Genetics, and Physiology.Cells. 2022 May 3;11(9):1529. doi: 10.3390/cells11091529. Cells. 2022. PMID: 35563835 Free PMC article. Review.
-
Plant and animal transglutaminases: do similar functions imply similar structures?Amino Acids. 2009 Apr;36(4):643-57. doi: 10.1007/s00726-008-0131-9. Epub 2008 Jul 12. Amino Acids. 2009. PMID: 18622667 Review.
-
Transglutaminase as polyamine mediator in plant growth and differentiation.Amino Acids. 2016 Oct;48(10):2467-78. doi: 10.1007/s00726-016-2235-y. Epub 2016 Apr 21. Amino Acids. 2016. PMID: 27101214 Review.
-
Suborganellar localisation and effect of light on Helianthus tuberosus chloroplast transglutaminases and their substrates.Planta. 2003 May;217(1):84-95. doi: 10.1007/s00425-003-0998-3. Epub 2003 Mar 14. Planta. 2003. PMID: 12721852
-
Transglutaminases of higher, lower plants and fungi.Prog Exp Tumor Res. 2005;38:223-47. doi: 10.1159/000084243. Prog Exp Tumor Res. 2005. PMID: 15746539 Review. No abstract available.
Cited by
-
Plant Transglutaminases: New Insights in Biochemistry, Genetics, and Physiology.Cells. 2022 May 3;11(9):1529. doi: 10.3390/cells11091529. Cells. 2022. PMID: 35563835 Free PMC article. Review.
-
Is now the time for a Rubiscuit or Ruburger? Increased interest in Rubisco as a food protein.J Exp Bot. 2023 Jan 11;74(2):627-637. doi: 10.1093/jxb/erac414. J Exp Bot. 2023. PMID: 36260435 Free PMC article.
-
Crystal structure and inhibition studies of transglutaminase from Streptomyces mobaraense.J Biol Chem. 2011 Mar 4;286(9):7301-7. doi: 10.1074/jbc.M110.203315. Epub 2010 Dec 29. J Biol Chem. 2011. PMID: 21193394 Free PMC article.
-
Dark-induced senescence of barley leaves involves activation of plastid transglutaminases.Amino Acids. 2015 Apr;47(4):825-38. doi: 10.1007/s00726-014-1912-y. Epub 2015 Jan 13. Amino Acids. 2015. PMID: 25583605 Free PMC article.
-
Cutin:cutin-acid endo-transacylase (CCT), a cuticle-remodelling enzyme activity in the plant epidermis.Biochem J. 2021 Feb 26;478(4):777-798. doi: 10.1042/BCJ20200835. Biochem J. 2021. PMID: 33511979 Free PMC article.
References
-
- Beninati S, Folk JE. Covalent polyamine-protein conjugates: analysis and distribution. Advances in Experimental Medicine and Biology. 1988;250:411–422. - PubMed
-
- Bernet E, Claparols I, Dondini L, Santos MA, Serafini-Fracassini D, Torné JM. Changes in polyamine content, arginine and ornithine decarboxylases and transglutaminase activities during light/dark phases of initial differentiation in maize calluses and their chloroplasts. Plant Physiology and Biochemistry. 1999;37:899–909.
-
- Bertossi F, Bagni N, Moruzzi G, Caldarera CM. Spermine as a new growth-promoting substance for Helianthus tuberosus (Jerusalem artichoke) in vitro. Experientia. 1965;21:81–82. - PubMed
-
- Besford RT, Richardson CM, Campos JL, Tiburcio AF. Effect of polyamines on stabilization of molecular complexes in thylakoid membranes of osmotically stressed oat leaves. Planta. 1993;189:201–206.
-
- Carvajal-Vallejos PK, Campos A, Fuentes-Prior P, Villalobos E, Almeida AM, Barberà E, et al. Purification and in vitro refolding of maize chloroplast transglutaminase over expressed in Escherichia coli. Biotechnology Letters. 2007;29:1255–1262. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

