Effects of once versus twice-daily parathyroid hormone 1-34 therapy in children with hypoparathyroidism
- PMID: 18492754
- PMCID: PMC2567852
- DOI: 10.1210/jc.2007-2552
Effects of once versus twice-daily parathyroid hormone 1-34 therapy in children with hypoparathyroidism
Abstract
Context: Hypoparathyroidism is among the few hormonal insufficiency states not treated with replacement of the missing hormone. Long-term conventional therapy with vitamin D and analogs may lead to nephrocalcinosis and renal insufficiency.
Objective: Our objective was to compare the response of once-daily vs. twice-daily PTH 1-34 treatment in children with hypoparathyroidism.
Setting: The study was conducted at a clinical research center.
Subjects: Fourteen children ages 4-17 yr with chronic hypoparathyroidism were studied.
Study design: This was a randomized cross-over trial, lasting 28 wk, which compared two dose regimens, once-daily vs. twice-daily PTH1-34. Each 14-wk study arm was divided into a 2-wk inpatient dose-adjustment phase and a 12-wk outpatient phase.
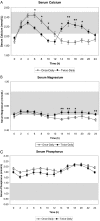
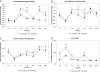
Results: Mean predose serum calcium was maintained at levels just below the normal range. Repeated serum measures over a 24-h period showed that twice-daily PTH 1-34 increased serum calcium and magnesium levels more effectively than a once-daily dose. This was especially evident during the second half of the day (12-24 h). PTH 1-34 normalized mean 24-h urine calcium excretion on both treatment schedules. This was achieved with half the PTH 1-34 dose during the twice-daily regimen compared with the once-daily regimen (twice-daily, 25 +/-15 microg/d vs. once-daily, 58 +/- 28 microg/d; P < 0.001).
Conclusions: We conclude that a twice-daily PTH 1-34 regimen provides a more effective treatment of hypoparathyroidism compared with once-daily treatment because it reduces the variation in serum calcium levels and accomplishes this at a lower total daily PTH 1-34 dose. The results showed, as in the previous study of adult patients with hypoparathyroidism, that a twice-daily regimen produced significantly improved metabolic control compared with once-daily PTH 1-34.
Figures
Comment in
-
Hypoparathyroidism: is it time for replacement therapy?J Clin Endocrinol Metab. 2008 Sep;93(9):3307-9. doi: 10.1210/jc.2008-1216. J Clin Endocrinol Metab. 2008. PMID: 18772463 Free PMC article. No abstract available.
Similar articles
-
A randomized, cross-over trial of once-daily versus twice-daily parathyroid hormone 1-34 in treatment of hypoparathyroidism.J Clin Endocrinol Metab. 1998 Oct;83(10):3480-6. doi: 10.1210/jcem.83.10.5185. J Clin Endocrinol Metab. 1998. PMID: 9768650 Clinical Trial.
-
Long-term treatment of 12 children with chronic hypoparathyroidism: a randomized trial comparing synthetic human parathyroid hormone 1-34 versus calcitriol and calcium.J Clin Endocrinol Metab. 2010 Jun;95(6):2680-8. doi: 10.1210/jc.2009-2464. Epub 2010 Apr 14. J Clin Endocrinol Metab. 2010. PMID: 20392870 Free PMC article. Clinical Trial.
-
Synthetic human parathyroid hormone 1-34 vs calcitriol and calcium in the treatment of hypoparathyroidism.JAMA. 1996 Aug 28;276(8):631-6. JAMA. 1996. PMID: 8773636 Clinical Trial.
-
Sporadic hypoparathyroidism treated with teriparatide: a case report and literature review.Exp Clin Endocrinol Diabetes. 2007 Jan;115(1):50-4. doi: 10.1055/s-2007-967088. Exp Clin Endocrinol Diabetes. 2007. PMID: 17286236 Review.
-
Advances in the treatment of hypoparathyroidism with PTH 1-34.Bone. 2019 Mar;120:535-541. doi: 10.1016/j.bone.2018.09.018. Epub 2018 Sep 21. Bone. 2019. PMID: 30243992 Review.
Cited by
-
Recent advances in understanding and managing hypoparathyroidism.F1000Res. 2020 Jul 23;9:F1000 Faculty Rev-766. doi: 10.12688/f1000research.22717.1. eCollection 2020. F1000Res. 2020. PMID: 32765831 Free PMC article. Review.
-
Diagnosis and treatment of hypoparathyroidism: a position statement from the Brazilian Society of Endocrinology and Metabolism.Arch Endocrinol Metab. 2018 Feb;62(1):106-124. doi: 10.20945/2359-3997000000015. Arch Endocrinol Metab. 2018. PMID: 29694629 Free PMC article.
-
A Randomized Double-Blind Placebo-Controlled First-In-Human Phase 1 Trial of TransCon PTH in Healthy Adults.J Bone Miner Res. 2020 Aug;35(8):1430-1440. doi: 10.1002/jbmr.4016. Epub 2020 Apr 16. J Bone Miner Res. 2020. PMID: 32212275 Free PMC article. Clinical Trial.
-
Continuous Subcutaneous Delivery of rhPTH(1-84) and rhPTH(1-34) by Pump in Adults With Hypoparathyroidism.J Endocr Soc. 2024 Mar 29;8(5):bvae053. doi: 10.1210/jendso/bvae053. eCollection 2024 Mar 12. J Endocr Soc. 2024. PMID: 38562130 Free PMC article.
-
Backbone modification of a polypeptide drug alters duration of action in vivo.Nat Biotechnol. 2014 Jul;32(7):653-5. doi: 10.1038/nbt.2920. Epub 2014 Jun 15. Nat Biotechnol. 2014. PMID: 24929976 Free PMC article.
References
-
- Winer KK, Yanovski JA, Cutler GB 1996 Synthetic human parathyroid hormone 1–34 vs calcitriol and calcium in the treatment of hypoparathyroidism. JAMA 276:631–636 - PubMed
-
- Winer KK, Yanovski JA, Sarani B, Cutler GB 1998 A randomized, cross-over trial of once-daily versus twice-daily parathyroid hormone 1–34 in treatment of hypoparathyroidism. J Clin Endocrinol Metab 83: 3480–3486 - PubMed
-
- Winer KK, Ko CW, Reynolds J, Dowdy K, Keil M, Peterson D Gerber L, McGarvey C, Cutler GB 2003 Long-term treatment of hypoparathyroidism: a randomized controlled study comparing parathyroid hormone-(1–34) versus calcitriol and calcium. J Clin Endocrinol Metab 88:4214–4220 - PubMed
-
- Chan JC, Young RB, Alon U, Mamunes P 1983 Hypercalcemia in children with disorders of calcium and phosphate metabolism during long-term treatment with 1,25-dihydroxyvitamin-D3. Pediatrics 72:225–233 - PubMed
-
- Chan JC, Young RB, Hartenberg MA, Chinchilli VM 1985 Calcium and phosphate metabolism in children with idiopathic hypoparathyroidism or pseudohypoparathyroidism: effects of 1,25-dihydroxyvitamin D3. J Pediatr 106:421–426 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

