Calpain activity and muscle wasting in sepsis
- PMID: 18492780
- PMCID: PMC2575901
- DOI: 10.1152/ajpendo.90226.2008
Calpain activity and muscle wasting in sepsis
Abstract
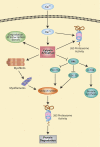
Muscle wasting in sepsis reflects activation of multiple proteolytic mechanisms, including lyosomal and ubiquitin-proteasome-dependent protein breakdown. Recent studies suggest that activation of the calpain system also plays an important role in sepsis-induced muscle wasting. Perhaps the most important consequence of calpain activation in skeletal muscle during sepsis is disruption of the sarcomere, allowing for the release of myofilaments (including actin and myosin) that are subsequently ubiquitinated and degraded by the 26S proteasome. Other important consequences of calpain activation that may contribute to muscle wasting during sepsis include degradation of certain transcription factors and nuclear cofactors, activation of the 26S proteasome, and inhibition of Akt activity, allowing for downstream activation of Foxo transcription factors and GSK-3beta. The role of calpain activation in sepsis-induced muscle wasting suggests that the calpain system may be a therapeutic target in the prevention and treatment of muscle wasting during sepsis. Furthermore, because calpain activation may also be involved in muscle wasting caused by other conditions, including different muscular dystrophies and cancer, calpain inhibitors may be beneficial not only in the treatment of sepsis-induced muscle wasting but in other conditions causing muscle atrophy as well.
Figures
References
-
- Agarkova I, Perriard JC. The M-band: an elastic web that crosslinks thick filaments in the center of the sarcomere. Trends Cell Biol 15: 477–485, 2005. - PubMed
-
- Aizawa H, Kawahara H, Tanaka K, Yokosawa H. Activation of the proteasome during Xenopus egg activation imploes a link between proteasome activation and intracellular calcium release. Biochem Biophys Res Commun 218: 224–228, 1996. - PubMed
-
- Auvin S, Pignol B, Navet E, Pons D, Marin JG, Bigg D, Chabrier PE. Novel dual inhibitors of calpain and lipid peroxidation. Bioorg Med Chem 14: 3825–3828, 2004. - PubMed
-
- Baar K, Nader G, Bodine S. Resistance exercise, muscle loading/unloading and the control of muscle mass. Essays Biochem 42: 61–74, 2006. - PubMed
-
- Baracos V, Greenberg RE, Goldberg AL. Influence of calcium and other divalent cations on protein turnover in rat skeletal muscle. Am J Physiol Endocrinol Metab 250: E702–E710, 1986. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

