Microbial production of plant benzylisoquinoline alkaloids
- PMID: 18492807
- PMCID: PMC2396723
- DOI: 10.1073/pnas.0802981105
Microbial production of plant benzylisoquinoline alkaloids
Abstract
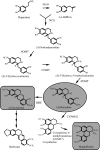
Benzylisoquinoline alkaloids, such as the analgesic compounds morphine and codeine, and the antibacterial agents berberine, palmatine, and magnoflorine, are synthesized from tyrosine in the Papaveraceae, Berberidaceae, Ranunculaceae, Magnoliaceae, and many other plant families. It is difficult to produce alkaloids on a large scale under the strict control of secondary metabolism in plants, and they are too complex for cost-effective chemical synthesis. By using a system that combines microbial and plant enzymes to produce desired benzylisoquinoline alkaloids, we synthesized (S)-reticuline, the key intermediate in benzylisoquinoline alkaloid biosynthesis, from dopamine by crude enzymes from transgenic Escherichia coli. The final yield of (S)-reticuline was 55 mg/liter within 1 h. Furthermore, we synthesized an aporphine alkaloid, magnoflorine, or a protoberberine alkaloid, scoulerine, from dopamine via reticuline by using different combination cultures of transgenic E. coli and Saccharomyces cerevisiae cells. The final yields of magnoflorine and scoulerine were 7.2 and 8.3 mg/liter culture medium. These results indicate that microbial systems that incorporate plant genes cannot only enable the mass production of scarce benzylisoquinoline alkaloids but may also open up pathways for the production of novel benzylisoquinoline alkaloids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Production platforms for the molecular pharming of alkaloid diversity.Proc Natl Acad Sci U S A. 2008 Jun 10;105(23):7897-8. doi: 10.1073/pnas.0803930105. Epub 2008 Jun 9. Proc Natl Acad Sci U S A. 2008. PMID: 18541922 Free PMC article. No abstract available.
Similar articles
-
Advancements in Microbial Cell Engineering for Benzylisoquinoline Alkaloid Production.ACS Synth Biol. 2024 Dec 20;13(12):3842-3856. doi: 10.1021/acssynbio.4c00599. Epub 2024 Nov 23. ACS Synth Biol. 2024. PMID: 39579377 Review.
-
Isolation and Characterization of O-methyltransferases Involved in the Biosynthesis of Glaucine in Glaucium flavum.Plant Physiol. 2015 Oct;169(2):1127-40. doi: 10.1104/pp.15.01240. Epub 2015 Aug 21. Plant Physiol. 2015. PMID: 26297140 Free PMC article.
-
Production of benzylisoquinoline alkaloids in Saccharomyces cerevisiae.Nat Chem Biol. 2008 Sep;4(9):564-73. doi: 10.1038/nchembio.105. Nat Chem Biol. 2008. PMID: 18690217 Free PMC article.
-
Isolation and Characterization of Reticuline N-Methyltransferase Involved in Biosynthesis of the Aporphine Alkaloid Magnoflorine in Opium Poppy.J Biol Chem. 2016 Nov 4;291(45):23416-23427. doi: 10.1074/jbc.M116.750893. Epub 2016 Sep 15. J Biol Chem. 2016. PMID: 27634038 Free PMC article.
-
Microbial Factories for the Production of Benzylisoquinoline Alkaloids.Trends Biotechnol. 2016 Mar;34(3):228-241. doi: 10.1016/j.tibtech.2015.12.005. Epub 2016 Jan 15. Trends Biotechnol. 2016. PMID: 26775900 Review.
Cited by
-
Engineering a microbial platform for de novo biosynthesis of diverse methylxanthines.Metab Eng. 2016 Nov;38:191-203. doi: 10.1016/j.ymben.2016.08.003. Epub 2016 Aug 9. Metab Eng. 2016. PMID: 27519552 Free PMC article.
-
Purine Permease-Type Benzylisoquinoline Alkaloid Transporters in Opium Poppy.Plant Physiol. 2019 Nov;181(3):916-933. doi: 10.1104/pp.19.00565. Epub 2019 Aug 29. Plant Physiol. 2019. PMID: 31467164 Free PMC article.
-
Advanced Strategies for Production of Natural Products in Yeast.iScience. 2020 Mar 27;23(3):100879. doi: 10.1016/j.isci.2020.100879. Epub 2020 Feb 1. iScience. 2020. PMID: 32087574 Free PMC article. Review.
-
Metabolic flux analysis of secondary metabolism in plants.Metab Eng Commun. 2020 Feb 1;10:e00123. doi: 10.1016/j.mec.2020.e00123. eCollection 2020 Jun. Metab Eng Commun. 2020. PMID: 32099803 Free PMC article. Review.
-
Biosynthesis of (R)-(-)-1-Octen-3-ol in Recombinant Saccharomyces cerevisiae with Lipoxygenase-1 and Hydroperoxide Lyase Genes from Tricholoma matsutake.J Microbiol Biotechnol. 2020 Feb 28;30(2):296-305. doi: 10.4014/jmb.2001.01049. J Microbiol Biotechnol. 2020. PMID: 32120462 Free PMC article.
References
-
- Allen RS, et al. RNAi-mediated replacement of morphine with the nonnarcotic alkaloid reticuline in opium poppy. Nat Biotechnol. 2004;22:1559–1566. - PubMed
-
- Hung TM, et al. Magnoflorine from Coptidis rhizoma protects high density lipoprotein during oxidant stress. Biol Pharm Bull. 2007;30:1157–1160. - PubMed
-
- Hung TM, et al. Protective effect of magnoflorine isolated from Coptidis rhizoma on Cu2+-induced oxidation of human low density lipoprotein. Planta Med. 2007;73:1281–1284. - PubMed
-
- Rashid MA, et al. Anti-HIV alkaloids from Toddalia asiatica. Nat Prod Res. 1995;6:153–156.
-
- Kong W, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–1351. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

