Effect of sulphation on the oestrogen agonist activity of the phytoestrogens genistein and daidzein in MCF-7 human breast cancer cells
- PMID: 18492816
- PMCID: PMC2386535
- DOI: 10.1677/JOE-07-0384
Effect of sulphation on the oestrogen agonist activity of the phytoestrogens genistein and daidzein in MCF-7 human breast cancer cells
Abstract
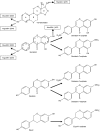
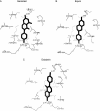
The phytoestrogens genistein, daidzein and the daidzein metabolite equol have been shown previously to possess oestrogen agonist activity. However, following consumption of soya diets, they are found in the body not only as aglycones but also as metabolites conjugated at their 4'- and 7-hydroxyl groups with sulphate. This paper describes the effects of monosulphation on the oestrogen agonist properties of these three phytoestrogens in MCF-7 human breast cancer cells in terms of their relative ability to compete with [(3)H]oestradiol for binding to oestrogen receptor (ER), to induce a stably transfected oestrogen-responsive reporter gene (ERE-CAT) and to stimulate cell growth. In no case did sulphation abolish activity. The 4'-sulphation of genistein reduced oestrogen agonist activity to a small extent in whole-cell assays but increased the relative binding affinity to ER. The 7-sulphation of genistein, and also of equol, reduced oestrogen agonist activity substantially in all assays. By contrast, the position of monosulphation of daidzein acted in an opposing manner on oestrogen agonist activity. Sulphation at the 4'-position of daidzein resulted in a modest reduction in oestrogen agonist activity but sulphation of daidzein at the 7-position resulted in an increase in oestrogen agonist activity. Molecular modelling and docking studies suggested that the inverse effects of sulphation could be explained by the binding of daidzein into the ligand-binding domain of the ER in the opposite orientation compared with genistein and equol. This is the first report of sulphation enhancing activity of an isoflavone and inverse effects of sulphation between individual phytoestrogens.
Figures


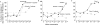


Similar articles
-
Phytoestrogens modulate binding response of estrogen receptors alpha and beta to the estrogen response element.J Agric Food Chem. 2003 Dec 17;51(26):7632-5. doi: 10.1021/jf034427b. J Agric Food Chem. 2003. PMID: 14664520
-
Comparative study of oestrogenic properties of eight phytoestrogens in MCF7 human breast cancer cells.J Steroid Biochem Mol Biol. 2005 Apr;94(5):431-43. doi: 10.1016/j.jsbmb.2004.12.041. Epub 2005 Mar 21. J Steroid Biochem Mol Biol. 2005. PMID: 15876408
-
Effects of dietary daidzein and its metabolite, equol, at physiological concentrations on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice.Carcinogenesis. 2006 Apr;27(4):856-63. doi: 10.1093/carcin/bgi320. Epub 2006 Jan 6. Carcinogenesis. 2006. PMID: 16399773
-
Phytoestrogens and breast cancer: a complex story.Inflammopharmacology. 2008 Oct;16(5):219-26. doi: 10.1007/s10787-008-8020-0. Inflammopharmacology. 2008. PMID: 18815740 Review.
-
Isoflavones, equol and cardiovascular disease: pharmacological and therapeutic insights.Curr Med Chem. 2007;14(26):2824-30. doi: 10.2174/092986707782360178. Curr Med Chem. 2007. PMID: 18045128 Review.
Cited by
-
Equol: A Bacterial Metabolite from The Daidzein Isoflavone and Its Presumed Beneficial Health Effects.Nutrients. 2019 Sep 16;11(9):2231. doi: 10.3390/nu11092231. Nutrients. 2019. PMID: 31527435 Free PMC article. Review.
-
Inhibition of cancer cell invasion and metastasis by genistein.Cancer Metastasis Rev. 2010 Sep;29(3):465-82. doi: 10.1007/s10555-010-9238-z. Cancer Metastasis Rev. 2010. PMID: 20730632 Free PMC article. Review.
-
Effects of isoflavones on breast tissue and the thyroid hormone system in humans: a comprehensive safety evaluation.Arch Toxicol. 2018 Sep;92(9):2703-2748. doi: 10.1007/s00204-018-2279-8. Epub 2018 Aug 21. Arch Toxicol. 2018. PMID: 30132047 Free PMC article. Review.
-
Therapeutic Potential of Isoflavones with an Emphasis on Daidzein.Oxid Med Cell Longev. 2021 Sep 9;2021:6331630. doi: 10.1155/2021/6331630. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34539970 Free PMC article. Review.
-
Exploring the In Vivo Existence Forms (23 Original Constituents and 147 Metabolites) of Astragali Radix Total Flavonoids and Their Distributions in Rats Using HPLC-DAD-ESI-IT-TOF-MSn.Molecules. 2020 Nov 26;25(23):5560. doi: 10.3390/molecules25235560. Molecules. 2020. PMID: 33256251 Free PMC article.
References
-
- Adlercreutz H, Mazur HW. Phyto-oestrogens and western diseases. Annals of Medicine. 1997;29:95–120. - PubMed
-
- Adlercreutz H, van der Wildt J, Kinzel J, Attalla H, Wahala K, Makela T, Hase T, Fotis T. Lignan and isoflavonoid conjugates in human urine. Journal of Steroid Biochemistry and Molecular Biology. 1995;52:97–103. - PubMed
-
- Brzozowski AM, Pike ACW, Dauter Z, Hubbard RE, Bonn T, Engstrom O, Ohman L, greene GL, Gustafsson JA, Carlquist M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–758. - PubMed
-
- Byford JR, Shaw LE, Drew MGB, Pope GS, Sauer MJ, Darbre PD. Oestrogenic activity of parabens in MCF7 human breast cancer cells. Journal of Steroid Biochemistry and Molecular Biology. 2002;80:49–60. - PubMed
-
- Carosati E, Sciabola S, Cruciani G. Hydrogen bonding interactions of covalently bonded fluorine atoms: from crystallographic data to a new angular function in the GRID force field. Journal of Medicinal Chemistry. 2004;47:5114–5125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

