Diversification of the core RNA interference machinery in Chlamydomonas reinhardtii and the role of DCL1 in transposon silencing
- PMID: 18493041
- PMCID: PMC2390644
- DOI: 10.1534/genetics.107.086546
Diversification of the core RNA interference machinery in Chlamydomonas reinhardtii and the role of DCL1 in transposon silencing
Abstract
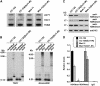
Small RNA-guided gene silencing is an evolutionarily conserved process that operates by a variety of molecular mechanisms. In multicellular eukaryotes, the core components of RNA-mediated silencing have significantly expanded and diversified, resulting in partly distinct pathways for the epigenetic control of gene expression and genomic parasites. In contrast, many unicellular organisms with small nuclear genomes seem to have lost entirely the RNA-silencing machinery or have retained only a basic set of components. We report here that Chlamydomonas reinhardtii, a unicellular eukaryote with a relatively large nuclear genome, has undergone extensive duplication of Dicer and Argonaute polypeptides after the divergence of the green algae and land plant lineages. Chlamydomonas encodes three Dicers and three Argonautes with DICER-LIKE1 (DCL1) and ARGONAUTE1 being more divergent than the other paralogs. Interestingly, DCL1 is uniquely involved in the post-transcriptional silencing of retrotransposons such as TOC1. Moreover, on the basis of the subcellular distribution of TOC1 small RNAs and target transcripts, this pathway most likely operates in the nucleus. However, Chlamydomonas also relies on a DCL1-independent, transcriptional silencing mechanism(s) for the maintenance of transposon repression. Our results suggest that multiple, partly redundant epigenetic processes are involved in preventing transposon mobilization in this green alga.
Figures



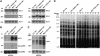

References
-
- Allen, E., Z. Xie, A. M. Gustafson and J. C. Carrington, 2005. microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121 207–221. - PubMed
-
- Aravin, A. A., G. J. Hannon and J. Brennecke, 2007. The Piwi-piRNA pathway provides an adaptive defense in the transposon arms race. Science 318 761–764. - PubMed
-
- Bartel, D. P., 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116 282–297. - PubMed
-
- Baulcombe, D., 2004. RNA silencing in plants. Nature 431 356–363. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

