Caenorhabditis elegans num-1 negatively regulates endocytic recycling
- PMID: 18493060
- PMCID: PMC2390616
- DOI: 10.1534/genetics.108.087247
Caenorhabditis elegans num-1 negatively regulates endocytic recycling
Abstract
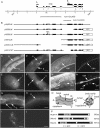
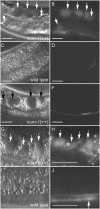
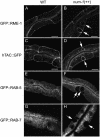
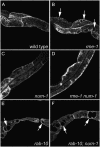
Much of the material taken into cells by endocytosis is rapidly returned to the plasma membrane by the endocytic recycling pathway. Although recycling is vital for the correct localization of cell membrane receptors and lipids, the molecular mechanisms that regulate recycling are only partially understood. Here we show that in Caenorhabditis elegans endocytic recycling is inhibited by NUM-1A, the nematode Numb homolog. NUM-1AGFP fusion protein is localized to the baso-lateral surfaces of many polarized epithelial cells, including the hypodermis and the intestine. We show that increased NUM-1A levels cause morphological defects in these cells similar to those caused by loss-of-function mutations in rme-1, a positive regulator of recycling in both C. elegans and mammals. We describe the isolation of worms lacking num-1A activity and show that, consistent with a model in which NUM-1A negatively regulates recycling in the intestine, loss of num-1A function bypasses the requirement for RME-1. Genetic epistasis analysis with rab-10, which is required at an early part of the recycling pathway, suggests that loss of num-1A function does not affect the uptake of material by endocytosis but rather inhibits baso-lateral recycling downstream of rab-10.
Figures



References
-
- Apodaca, G., C. Enrich and K. E. Mostov, 1994. The calmodulin antagonist, W-13, alters transcytosis, recycling, and the morphology of the endocytic pathway in Madin-Darby canine kidney cells. J. Biol. Chem. 269 19005–19013. - PubMed
-
- Balklava, Z., S. Pant, H. Fares and B. D. Grant, 2007. Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat. Cell Biol. 9 1066–1073. - PubMed
-
- Berdnik, D., T. Torok, M. Gonzalez-Gaitan and J. A. Knoblich, 2002. The endocytic protein α-Adaptin is required for numb-mediated asymmetric cell division in Drosophila. Dev. Cell 3 221–231. - PubMed
-
- Brodsky, F. M., C. Y. Chen, C. Knuehl, M. C. Towler and D. E. Wakeham, 2001. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 17 517–568. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

