Fetal programming of adrenal androgen excess: lessons from a nonhuman primate model of polycystic ovary syndrome
- PMID: 18493139
- PMCID: PMC2531212
- DOI: 10.1159/000134831
Fetal programming of adrenal androgen excess: lessons from a nonhuman primate model of polycystic ovary syndrome
Abstract
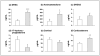
Adrenal androgen excess is found in adult female rhesus monkeys previously exposed to androgen treatment during early gestation. In adulthood, such prenatally androgenized female monkeys exhibit elevated basal circulating levels of dehydroepiandrosterone sulfate (DHEAS), typical of polycystic ovary syndrome (PCOS) women with adrenal androgen excess. Further androgen and glucocorticoid abnormalities in PA female monkeys are revealed by acute ACTH stimulation: DHEA, androstenedione and corticosterone responses are all elevated compared to responses in controls. Pioglitazone treatment, however, diminishes circulating DHEAS responses to ACTH in both prenatally androgenized and control female monkeys, while increasing the 17-hydroxyprogesterone response and reducing the DHEA to 17-hydroxyprogesterone ratio. Since 60-min post-ACTH serum values for 17-hydroxyprogesterone correlate negatively with basal serum insulin levels (all female monkeys on pioglitazone and placebo treatment combined), while similar DHEAS values correlate positively with basal serum insulin levels, circulating insulin levels may preferentially support adrenal androgen biosynthesis in both prenatally androgenized and control female rhesus monkeys. Overall, our findings suggest that differentiation of the monkey adrenal cortex in a hyperandrogenic fetal environment may permanently upregulate adult adrenal androgen biosynthesis through specific elevation of 17,20-lyase activity in the zona fasciculata-reticularis. As adult prenatally androgenized female rhesus monkeys closely emulate PCOS-like symptoms, excess fetal androgen programming may contribute to adult adrenal androgen excess in women with PCOS.
Figures


References
-
- Conley AJ, Bird IM. The role of cytochrome P450 17 alpha-hydroxylase and 3 beta-hydroxysteroid dehydrogenase in the integration of gonadal and adrenal steroidogenesis via the delta 5 and delta 4 pathways of steroidogenesis in mammals. Biol Reprod. 1997;56:789–799. - PubMed
-
- Conley AJ, Pattison JC, Bird IM. Variations in adrenal androgen production among (nonhuman) primates. Semin Reprod Med. 2004;22:311–326. - PubMed
-
- Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev. 1997;18:378–403. - PubMed
-
- Mapes S, Tarantal AF, Parker CR, Moran FM, Bahr JM, Pyter L, Conley AJ. Adrenocortical cytochrome b5 expression during fetal development of the rhesus macaque. Endocrinology. 2002;143:1451–1458. - PubMed
-
- Conley AJ. On the zonation of the adrenal cortex. In: Flueck CE, Miller WL, editors. Disorders of the Human Adrenal Cortex. Basel: Karger; 2008. pp. 000–000.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

