Chronic inhibition of the Na+/H+ - exchanger causes regression of hypertrophy, heart failure, and ionic and electrophysiological remodelling
- PMID: 18493245
- PMCID: PMC2483399
- DOI: 10.1038/bjp.2008.189
Chronic inhibition of the Na+/H+ - exchanger causes regression of hypertrophy, heart failure, and ionic and electrophysiological remodelling
Abstract
Background and purpose: Increased activity of the Na+/H+ -exchanger (NHE-1) in heart failure underlies raised [Na+]i causing disturbances of calcium handling. Inhibition of NHE-1, initiated at the onset of pressure/volume overload, prevents development of hypertrophy, heart failure and remodelling. We hypothesized that chronic inhibition of NHE-1, initiated at a later stage, would induce regression of hypertrophy, heart failure, and ionic and electrophysiological remodelling.
Experimental approach: Development of heart failure in rabbits was monitored electrocardiographically and echocardiographically, after one or three months. Cardiac myocytes were also isolated. One group of animals were treated with cariporide (inhibitor of NHE-1) in the diet after one month. Cytoplasmic calcium, sodium and action potentials were measured with fluorescent markers and sarcoplasmic reticulum calcium content by rapid cooling. Calcium after-transients were elicited after rapid pacing. Sodium channel current (INa) was measured using patch-clamp techniques.
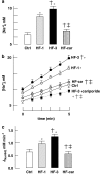
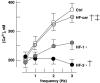
Key results: Hypertrophy and heart failure developed after one month and progressed during the next two months. After one month, dietary treatment with cariporide was initiated. Two months of treatment reduced hypertrophy and heart failure, duration of action potential QT-interval and QRS, and restored sodium and calcium handling and the incidence of calcium after-transients. In cardiac myocytes, parameters of INa were not changed by cariporide.
Conclusion and implications: In rabbit hearts with hypertrophy and signs of heart failure one month after induction of pressure/volume overload, two months of dietary treatment with the NHE-1 inhibitor cariporide caused regression of hypertrophy, heart failure and ionic and electrophysiological remodelling.
Figures






References
-
- Aiello EA, Villa-Abrille MC, Dulce RA, Cingolani HE, Perz NG. Endothelin-1 stimulates the Na+/Ca2+-exchanger reverse mode through intracellular Na+ (Na+i)-dependent pathways. Hypertension. 2005;45:288–243. - PubMed
-
- Baartscheer A. Chronic inhibition of the Na+/H+-exchanger in the heart. Curr Vasc Pharmacol. 2006;4:23–29. - PubMed
-
- Baartscheer A, Borren v MMC, Schumacher CA, Belterman CNW, Coronel R, Fiolet JWT. Increased Na+/H+-exchange activity is the cause of increased [Na+]i in heart failure and underlies disturbed calcium handling. Cardiovasc Res. 2003b;57:1015–1024. - PubMed
-
- Baartscheer A, Schumacher CA, Belterman CNW, Coronel R, Fiolet JWT. [Na+]i and the driving force of the Na+/Ca2+-exchanger in heart failure. Cardiovasc Res. 2003a;57:986–995. - PubMed
-
- Baartscheer A, Schumacher CA, Belterman CNW, Coronel R, Fiolet JWT. SR calcium handling and calcium after-transients in a rabbit model of heart failure. Cardiovasc Res. 2003c;58:99–108. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

