A multi-center randomised controlled trial of gatifloxacin versus azithromycin for the treatment of uncomplicated typhoid fever in children and adults in Vietnam
- PMID: 18493312
- PMCID: PMC2374894
- DOI: 10.1371/journal.pone.0002188
A multi-center randomised controlled trial of gatifloxacin versus azithromycin for the treatment of uncomplicated typhoid fever in children and adults in Vietnam
Abstract
Background: Drug resistant typhoid fever is a major clinical problem globally. Many of the first line antibiotics, including the older generation fluoroquinolones, ciprofloxacin and ofloxacin, are failing.
Objectives: We performed a randomised controlled trial to compare the efficacy and safety of gatifloxacin (10 mg/kg/day) versus azithromycin (20 mg/kg/day) as a once daily oral dose for 7 days for the treatment of uncomplicated typhoid fever in children and adults in Vietnam.
Methods: An open-label multi-centre randomised trial with pre-specified per protocol analysis and intention to treat analysis was conducted. The primary outcome was fever clearance time, the secondary outcome was overall treatment failure (clinical or microbiological failure, development of typhoid fever-related complications, relapse or faecal carriage of S. typhi).
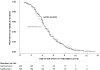
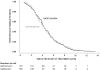
Principal findings: We enrolled 358 children and adults with suspected typhoid fever. There was no death in the study. 287 patients had blood culture confirmed typhoid fever, 145 patients received gatifloxacin and 142 patients received azithromycin. The median FCT was 106 hours in both treatment arms (95% Confidence Interval [CI]; 94-118 hours for gatifloxacin versus 88-112 hours for azithromycin), (logrank test p = 0.984, HR [95% CI] = 1.0 [0.80-1.26]). Overall treatment failure occurred in 13/145 (9%) patients in the gatifloxacin group and 13/140 (9.3%) patients in the azithromycin group, (logrank test p = 0.854, HR [95% CI] = 0.93 [0.43-2.0]). 96% (254/263) of the Salmonella enterica serovar Typhi isolates were resistant to nalidixic acid and 58% (153/263) were multidrug resistant.
Conclusions: Both antibiotics showed an excellent efficacy and safety profile. Both gatifloxacin and azithromycin can be recommended for the treatment of typhoid fever particularly in regions with high rates of multidrug and nalidixic acid resistance. The cost of a 7-day treatment course of gatifloxacin is approximately one third of the cost of azithromycin in Vietnam.
Trial registration: Controlled-Trials.com ISRCTN67946944.
Conflict of interest statement
Figures



References
-
- Bhan MK, Bahl R, Bhatnagar S. Typhoid and paratyphoid fever. Lancet. 2005;366:749–762. - PubMed
-
- Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid fever. N Engl J Med. 2002;347:1770–1782. - PubMed
-
- Nguyen TA, Ha Ba K, Nguyen TD. [Typhoid fever in South Vietnam, 1990–1993]. Bull Soc Pathol Exot. 1993;86:476–478. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

