Replicative senescence of mesenchymal stem cells: a continuous and organized process
- PMID: 18493317
- PMCID: PMC2374903
- DOI: 10.1371/journal.pone.0002213
Replicative senescence of mesenchymal stem cells: a continuous and organized process
Abstract
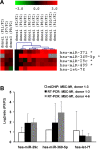
Mesenchymal stem cells (MSC) comprise a promising tool for cellular therapy. These cells are usually culture expanded prior to their application. However, a precise molecular definition of MSC and the sequel of long-term in vitro culture are yet unknown. In this study, we have addressed the impact of replicative senescence on human MSC preparations. Within 43 to 77 days of cultivation (7 to 12 passages), MSC demonstrated morphological abnormalities, enlargement, attenuated expression of specific surface markers, and ultimately proliferation arrest. Adipogenic differentiation potential decreased whereas the propensity for osteogenic differentiation increased. mRNA expression profiling revealed a consistent pattern of alterations in the global gene expression signature of MSC at different passages. These changes are not restricted to later passages, but are continuously acquired with increasing passages. Genes involved in cell cycle, DNA replication and DNA repair are significantly down-regulated in late passages. Genes from chromosome 4q21 were over-represented among differentially regulated transcripts. Differential expression of 10 genes has been verified in independent donor samples as well as in MSC that were isolated under different culture conditions. Furthermore, miRNA expression profiling revealed an up-regulation of hsa-mir-371, hsa-mir-369-5P, hsa-mir-29c, hsa-mir-499 and hsa-let-7f upon in vitro propagation. Our studies indicate that replicative senescence of MSC preparations is a continuous process starting from the first passage onwards. This process includes far reaching alterations in phenotype, differentiation potential, global gene expression patterns, and miRNA profiles that need to be considered for therapeutic application of MSC preparations.
Conflict of interest statement
Figures





References
-
- Reyes M, Lund T, Lenvik T, Aguiar D, Koodie L, et al. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98:2615–2625. - PubMed
-
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. - PubMed
-
- Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. - PubMed
-
- Horwitz EM, Le BK, Dominici M, Mueller I, Slaper-Cortenbach I, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

