Duration of protection against malaria and anaemia provided by intermittent preventive treatment in infants in Navrongo, Ghana
- PMID: 18493597
- PMCID: PMC2375060
- DOI: 10.1371/journal.pone.0002227
Duration of protection against malaria and anaemia provided by intermittent preventive treatment in infants in Navrongo, Ghana
Abstract
Background: Intermittent preventive treatment for malaria in Infants (IPTi) has been shown to give effective and safe protection against malaria. It has been suggested that IPTi might have long-lasting beneficial effects but, in most settings, the protection provided by IPTi appears to be short-lived. Knowledge of the duration of protection given by IPTi would help interpret the results of existing trials and suggest optimal delivery schedules for IPTi. This study investigated how the protective efficacy of IPTi against malaria and anaemia changes over time.
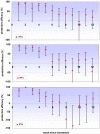
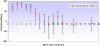
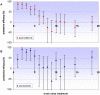
Methods and findings: A secondary analysis of data from a cluster-randomised, placebo-controlled trial of IPTi using sulfadoxine-pyrimethamine (SP) in Ghana was conducted. In this trial IPTi was given to 2485 infants at 3, 4, 9 and 12 months of age; children remained in follow-up until two years of age. Poisson regression with a random effect to adjust for the cluster-randomised design was used to determine protective efficacy of IPTi against clinical malaria and anaemia in defined time strata following administration of IPTi. Analysis of first-or-only clinical malaria episode following the individual IPTi doses showed that some protection against malaria lasted between 4 to 6 weeks. A similar pattern was seen when the incidence of all malaria episodes up to 2 years of age was analysed in relation to the most recent IPT, by pooling the incidence of malaria after the individual IPTi doses. Protective efficacy within four weeks of IPTi was 75.2% (95% CI: 66-82) against malaria, 78.9% (95% CI: 69-86) against high parasite density malaria, and 93.8% (95% CI: 73-99) against anaemia. Protection against these outcomes was short-lived, with evidence of any effect lasting for only 6, 6 and 4 weeks respectively. Protection in children who were parasitaemic when receiving IPTi appeared to be of shorter duration than in uninfected children. There was no evidence of any benefit of IPTi after the immediate period following the IPTi doses.
Conclusions: Intermittent preventive treatment provides considerable protection against malaria and anaemia for short periods, even in an area of intense seasonal transmission. Due to the relatively short duration of protection provided by each dose of IPTi, this treatment will be of most benefit when delivered at the time of peak malaria incidence.
Conflict of interest statement
Figures



References
-
- Schellenberg D, Menendez C, Kahigwa E, Aponte J, Vidal J, et al. Intermittent treatment for malaria and anaemia control at time of routine vaccinations in Tanzanian infants: a randomised, placebo-controlled trial. Lancet. 2001;357:1471–1477. - PubMed
-
- Schellenberg D, Menendez C, Aponte JJ, Kahigwa E, Tanner M, et al. Intermittent preventive antimalarial treatment for Tanzanian infants: follow-up to age 2 years of a randomised, placebo-controlled trial. Lancet. 2005;365:1481–1483. - PubMed
-
- Cisse B, Sokhna C, Boulanger D, Milet J, Ba EH, et al. Seasonal intermittent preventive treatment with artesunate and sulfadoxine-pyrimethamine for prevention of malaria in Senegalese children: a randomised, placebo-controlled, double-blind trial. Lancet. 2006;367:659–667. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

