A murine model of falciparum-malaria by in vivo selection of competent strains in non-myelodepleted mice engrafted with human erythrocytes
- PMID: 18493601
- PMCID: PMC2375113
- DOI: 10.1371/journal.pone.0002252
A murine model of falciparum-malaria by in vivo selection of competent strains in non-myelodepleted mice engrafted with human erythrocytes
Abstract
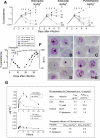
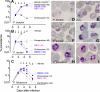
To counter the global threat caused by Plasmodium falciparum malaria, new drugs and vaccines are urgently needed. However, there are no practical animal models because P. falciparum infects human erythrocytes almost exclusively. Here we describe a reliable falciparum murine model of malaria by generating strains of P. falciparum in vivo that can infect immunodeficient mice engrafted with human erythrocytes. We infected NOD(scid/beta2m-/-) mice engrafted with human erythrocytes with P. falciparum obtained from in vitro cultures. After apparent clearance, we obtained isolates of P. falciparum able to grow in peripheral blood of engrafted NOD(scid/beta2m-/-) mice. Of the isolates obtained, we expanded in vivo and established the isolate Pf3D7(0087/N9) as a reference strain for model development. Pf3D7(0087/N9) caused productive persistent infections in 100% of engrafted mice infected intravenously. The infection caused a relative anemia due to selective elimination of human erythrocytes by a mechanism dependent on parasite density in peripheral blood. Using this model, we implemented and validated a reproducible assay of antimalarial activity useful for drug discovery. Thus, our results demonstrate that P. falciparum contains clones able to grow reproducibly in mice engrafted with human erythrocytes without the use of myeloablative methods.
Conflict of interest statement
Figures





References
-
- Greenwood BM, Bojang K, Whitty CJM, Targett GAT. Malaria. Lancet. 2005;365:1487–1498. - PubMed
-
- Gysin J. Animal models: Primates. In: Sherman IW, editor. Malaria: Parasite biology, pathogenesis and protection. Washington, D.C.: ASM Press; 1998. pp. 419–441.
-
- Fidock DA, Rosenthal PJ, Croft SL, Brun R, Nwaka S. Antimalarial drug discovery: efficacy models for compound screening. Nat Rev Drug Discov. 2004;3:509–520. - PubMed
-
- Landau I, Gautret P. Animal models: Rodents. In: Sherman IW, editor. Malaria: parasite biology, pathogenesis and protection. Washington, D.C.: ASM Press; 1998. pp. 401–417.
-
- Carlton JM, Angiuoli SV, Suh BB, Kooij TW, Pertea M, et al. Genome sequence and comparative analysis of the model rodent malaria parasite Plasmodium yoelii yoelii. Nature. 2002;419:512–519. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

