S100B induces tau protein hyperphosphorylation via Dickopff-1 up-regulation and disrupts the Wnt pathway in human neural stem cells
- PMID: 18494933
- PMCID: PMC3538024
- DOI: 10.1111/j.1582-4934.2008.00159.x
S100B induces tau protein hyperphosphorylation via Dickopff-1 up-regulation and disrupts the Wnt pathway in human neural stem cells
Abstract
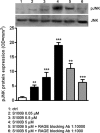
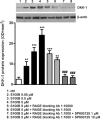
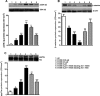
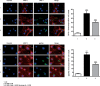
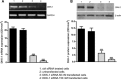
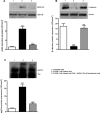
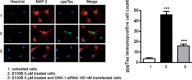
Previous studies suggest that levels of the astrocyte-derived S100B protein, such as those occurring in brain extra-cellular spaces consequent to persistent astroglial activation, may have a pathogenetic role in Alzheimer's disease (AD). Although S100B was reported to promote beta amyloid precursor protein overexpression, no clear mechanistic relationship between S100B and formation of neurofibrillary tangles (NFTs) is established. This in vitro study has been aimed at investigating whether S100B is able to disrupt Wnt pathway and lead to tau protein hyperphosphorylation. Utilizing Western blot, electrophoretic mobility shift assay, supershift and reverse transcriptase-polymerase chain reaction techniques, it has been demonstrated that micromolar S100B concentrations stimulate c-Jun N-terminal kinase (JNK) phosphorylation through the receptor for advanced glycation ending products, and subsequently activate nuclear AP-1/cJun transcription, in cultured human neural stem cells. In addition, as revealed by Western blot, small interfering RNA and immunofluorescence analysis, S100B-induced JNK activation increased expression of Dickopff-1 that, in turn, promoted glycogen synthase kinase 3beta phosphorylation and beta-catenin degradation, causing canonical Wnt pathway disruption and tau protein hyperphosphorylation. These findings propose a previously unrecognized link between S100B and tau hyperphosphorylation, suggesting S100B can contribute to NFT formation in AD and in all other conditions in which neuroinflammation may have a crucial role.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

