Ventricular assist device implant (AB 5000) prototype cannula: in vitro assessment of MRI issues at 3-Tesla
- PMID: 18495028
- PMCID: PMC2440740
- DOI: 10.1186/1532-429X-10-23
Ventricular assist device implant (AB 5000) prototype cannula: in vitro assessment of MRI issues at 3-Tesla
Abstract
Purpose: To evaluate MRI issues at 3-Tesla for a ventricular assist device (VAD).
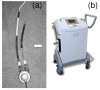
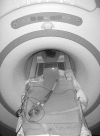
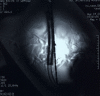
Methods: The AB5000 Ventricle with a prototype Nitinol wire-reinforced In-Flow Cannula and Out-Flow Cannula attached (Abiomed, Inc., Danvers, MA) was evaluated for magnetic field interactions, heating, and artifacts at 3-Tesla. MRI-related heating was assessed with the device in a gelled-saline-filled, head/torso phantom using a transmit/received RF body coil while performing MRI at a whole body averaged SAR of 3-W/kg for 15-min. Artifacts were assessed for the main metallic component of this VAD (atrial cannula) using T1-weighted, spin echo and gradient echo pulse sequences.
Results: The AB5000 Ventricle with the prototype In-Flow Cannula and Out-Flow Cannula attached showed relatively minor magnetic field interactions that will not cause movement in situ. Heating was not excessive (highest temperature change, +0.8 degrees C). Artifacts may create issues for diagnostic imaging if the area of interest is in the same area or close to the implanted metallic component of this VAD (i.e., the venous cannula).
Conclusion: The results of this investigation demonstrated that it would be acceptable for a patient with this VAD (AB5000 Ventricle with a prototype Nitinol wire-reinforced In-Flow Cannula and Out-Flow Cannula attached) to undergo MRI at 3-Tesla or less. Notably, it is likely that the operation console for this device requires positioning a suitable distance (beyond the 100 Gauss line or in the MR control room) from the 3-Tesla MR system to ensure proper function of the VAD.
Figures
References
-
- Levine GN, Gomes AS, Arai AE, Bluemke DA, Flamm SD, et al. Safety of magnetic resonance imaging in patients with cardiovascular devices: an American Heart Association scientific statement from the Committee on Diagnostic and Interventional Cardiac Catheterization. Circulation. 2007;116:2878–91. doi: 10.1161/CIRCULATIONAHA.107.187256. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

