Isoflurane preconditioning reduces mouse microglial activation and injury induced by lipopolysaccharide and interferon-gamma
- PMID: 18495358
- PMCID: PMC2492758
- DOI: 10.1016/j.neuroscience.2008.04.013
Isoflurane preconditioning reduces mouse microglial activation and injury induced by lipopolysaccharide and interferon-gamma
Abstract
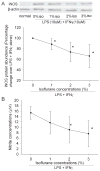
Activation and injury of microglial cells are involved in a broad range of brain diseases including stroke, brain infection and neurodegenerative diseases. However, there is very little information regarding how to reduce microglial reaction and preserve these cells to provide neuroprotection. Here, we showed that the incubation of C8-B4 mouse microglial cells with lipopolysaccharide (LPS) plus interferon-gamma (IFNgamma) for 24 h decreased the viability of these cells. Pretreatment of these cells with 1%, 2% or 3% isoflurane, a commonly used volatile anesthetic, for 1 h at 30 min before the exposure to LPS plus IFNgamma attenuated the reduction of cell viability (preconditioning effect). LPS plus IFNgamma also activated these microglial cells to express inducible nitric oxide synthase (iNOS) and to induce accumulation of nitrite, a stable oxidation product of nitric oxide, in the incubation medium. Isoflurane preconditioning attenuated these LPS plus IFNgamma effects on the iNOS expression and nitrite accumulation. Aminoguanidine, an iNOS inhibitor, attenuated the LPS plus IFNgamma-induced glutamate release and decrease of microglial viability. Isoflurane preconditioning also reduced LPS plus IFNgamma-induced glutamate release. Exogenous glutamate decreased microglial viability. Finally, the isoflurane preconditioning-induced protection was abolished by chelerythrine, a protein kinase C inhibitor. These results suggest that LPS plus IFNgamma activates the iNOS-nitric oxide-glutamate pathway to induce microglial injury and that this activation is attenuated by isoflurane preconditioning. Protein kinase C may be involved in the isoflurane preconditioning effects.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

