Interleukin-1 inhibits osmotically induced calcium signaling and volume regulation in articular chondrocytes
- PMID: 18495501
- PMCID: PMC3217044
- DOI: 10.1016/j.joca.2008.04.003
Interleukin-1 inhibits osmotically induced calcium signaling and volume regulation in articular chondrocytes
Abstract
Objective: Articular chondrocytes respond to osmotic stress with transient changes in cell volume and the intracellular concentration of calcium ion ([Ca(2+)](i)). The goal of this study was to examine the hypothesis that interleukin-1 (IL-1), a pro-inflammatory cytokine associated with osteoarthritis, influences osmotically induced Ca(2+) signaling.
Methods: Fluorescence ratio imaging was used to measure [Ca(2+)](i) and cell volume in response to hypo- or hyper-osmotic stress in isolated porcine chondrocytes, with or without pre-exposure to 10-ng/ml IL-1alpha. Inhibitors of IL-1 (IL-1 receptor antagonist, IL-1Ra), Ca(2+) mobilization (thapsigargin, an inhibitor of Ca-ATPases), and cytoskeletal remodeling (toxin B, an inhibitor of the Rho family of small GTPases) were used to determine the mechanisms involved in increased [Ca(2+)](i), F-actin remodeling, volume adaptation and active volume recovery.
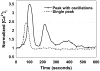
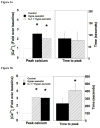
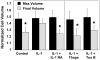
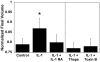
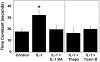
Results: In response to osmotic stress, chondrocytes exhibited transient increases in [Ca(2+)](i), generally followed by decaying oscillations. Pre-exposure to IL-1 significantly inhibited regulatory volume decrease (RVD) following hypo-osmotic swelling and reduced the change in cell volume and the time to peak [Ca(2+)](i) in response to hyper-osmotic stress, but did not affect the peak magnitudes of [Ca(2+)](i) in those cells that did respond. Co-treatment with IL-1Ra, thapsigargin, or toxin B restored these responses to control levels. The effects were associated with alterations in F-actin organization.
Conclusions: IL-1 alters the normal volumetric and Ca(2+) signaling response of chondrocytes to osmotic stress through mechanisms involving F-actin remodeling via small Rho GTPases. These findings provide further insights into the mechanisms by which IL-1 may interfere with normal physiologic processes in the chondrocyte, such as the adaptation or regulatory responses to mechanical or osmotic loading.
Figures


Similar articles
-
Effects of interleukin-1 on calcium signaling and the increase of filamentous actin in isolated and in situ articular chondrocytes.Arthritis Rheum. 2006 Jul;54(7):2164-74. doi: 10.1002/art.21941. Arthritis Rheum. 2006. PMID: 16802354
-
Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes.Arthritis Rheum. 2009 Oct;60(10):3028-37. doi: 10.1002/art.24799. Arthritis Rheum. 2009. PMID: 19790068 Free PMC article.
-
Hyper-osmotic stress induces volume change and calcium transients in chondrocytes by transmembrane, phospholipid, and G-protein pathways.J Biomech. 2001 Dec;34(12):1527-35. doi: 10.1016/s0021-9290(01)00156-7. J Biomech. 2001. PMID: 11716854
-
Effects of shear stress on articular chondrocyte metabolism.Biorheology. 2000;37(1-2):95-107. Biorheology. 2000. PMID: 10912182 Review.
-
Mechanosensory and mechanotransductive processes mediated by ion channels in articular chondrocytes: Potential therapeutic targets for osteoarthritis.Channels (Austin). 2021 Dec;15(1):339-359. doi: 10.1080/19336950.2021.1903184. Channels (Austin). 2021. PMID: 33775217 Free PMC article. Review.
Cited by
-
Compressive mechanical stress enhances susceptibility to interleukin-1 by increasing interleukin-1 receptor expression in 3D-cultured ATDC5 cells.BMC Musculoskelet Disord. 2021 Mar 1;22(1):238. doi: 10.1186/s12891-021-04095-x. BMC Musculoskelet Disord. 2021. PMID: 33648469 Free PMC article.
-
Physicochemical and biomechanical stimuli in cell-based articular cartilage repair.Curr Rheumatol Rep. 2015 Mar;17(3):22. doi: 10.1007/s11926-014-0493-9. Curr Rheumatol Rep. 2015. PMID: 25828845 Free PMC article. Review.
-
Dependence of zonal chondrocyte water transport properties on osmotic environment.Cell Mol Bioeng. 2008 Dec 1;1(4):339-348. doi: 10.1007/s12195-008-0026-6. Cell Mol Bioeng. 2008. PMID: 20011231 Free PMC article.
-
Activation of the mechanosensitive ion channels TRPV4 and PIEZO1 downregulates key regulatory systems in the chondrocyte mechanome.Connect Tissue Res. 2025 Jul;66(4):239-262. doi: 10.1080/03008207.2025.2498512. Epub 2025 May 21. Connect Tissue Res. 2025. PMID: 40395084
-
A Roadmap of In Vitro Models in Osteoarthritis: A Focus on Their Biological Relevance in Regenerative Medicine.J Clin Med. 2021 Apr 28;10(9):1920. doi: 10.3390/jcm10091920. J Clin Med. 2021. PMID: 33925222 Free PMC article. Review.
References
-
- Griffin TM, Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev. 2005;33:195–200. - PubMed
-
- Grodzinsky AJ, Levenston ME, Jin M, Frank EH. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2000;2:691–713. - PubMed
-
- Guilak F, Sah R, Setton L. Physical regulation of cartilage metabolism. In: Hayes W, Mow V, editors. Basic Orthopaedic Biomechanics. Philadelphia: Lippincott-Raven; 1997. pp. 179–207.
-
- Mow VC, Wang CC, Hung CT. The extracellular matrix, interstitial fluid and ions as a mechanical signal transducer in articular cartilage. Osteoarthritis Cartilage. 1999;7:41–58. - PubMed
-
- Guilak F, Mow VC. The mechanical environment of the chondrocyte: a biphasic finite element model of cell-matrix interactions in articular cartilage. J Biomech. 2000;33:1663–1673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

