Identification and characterization of a plastid-localized Arabidopsis glyoxylate reductase isoform: comparison with a cytosolic isoform and implications for cellular redox homeostasis and aldehyde detoxification
- PMID: 18495639
- PMCID: PMC2423656
- DOI: 10.1093/jxb/ern123
Identification and characterization of a plastid-localized Arabidopsis glyoxylate reductase isoform: comparison with a cytosolic isoform and implications for cellular redox homeostasis and aldehyde detoxification
Abstract
Enzymes that reduce the aldehyde chemical grouping (i.e. H-C=O) to its corresponding alcohol could be crucial in maintaining plant health. Recently, recombinant expression of a cytosolic enzyme from Arabidopsis thaliana (L.) Heynh (designated as glyoxylate reductase 1 or AtGR1) revealed that it effectively catalyses the in vitro reduction of both glyoxylate and succinic semialdehyde (SSA). In this paper, web-based bioinformatics tools revealed a second putative GR cDNA (GenBank Accession No. AAP42747; designated herein as AtGR2) that is 57% identical on an amino acid basis to GR1. Sequence encoding a putative targeting signal (N-terminal 43 amino acids) was deleted from the full-length GR2 cDNA and the resulting truncated gene was co-expressed with the molecular chaperones GroES/EL in Escherichia coli, enabling production and purification of soluble recombinant protein. Kinetic analysis revealed that recombinant GR2 catalysed the conversion of glyoxylate to glycolate (K(m) glyoxylate=34 microM), and SSA to gamma-hydroxybutyrate (K(m) SSA=8.96 mM) via an essentially irreversible, NADPH-based mechanism. GR2 had a 350-fold higher preference for glyoxylate than SSA, based on the performance constants (k(cat)/K(m)). Fluorescence microscopic analysis of tobacco (Nicotiana tabacum L.) suspension cells transiently transformed with GR1 linked to the green fluorescent protein (GFP) revealed that GR1 was localized to the cytosol, whereas GR2-GFP was localized to plastids via targeting information contained within its N-terminal 45 amino acids. The identification and characterization of distinct plastidial and cytosolic glyoxylate reductase isoforms is discussed with respect to aldehyde detoxification and the plant stress response.
Figures

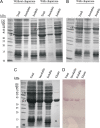
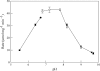


References
-
- Allan WL, Peiris C, Bown AW, Shelp BJ. Gamma-hydroxybutyrate accumulates in green tea leaves and soybean sprouts in response to oxygen deficiency. Canadian Journal of Plant Science. 2003a;83:951–953.
-
- Allan WL, Smith R, Shelp BJ. Application Bulletin AB-0015. Mississauga, ON, Canada: Agilent Technologies Inc.; 2003b. Direct measurement of γ-hydroxybutyrate (GHB) in crude plant extracts by liquid chromatography/negative ion-ES mass spectrometry; p. 4.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

