Gamma-hydroxybutyrate accumulation in Arabidopsis and tobacco plants is a general response to abiotic stress: putative regulation by redox balance and glyoxylate reductase isoforms
- PMID: 18495640
- PMCID: PMC2423657
- DOI: 10.1093/jxb/ern122
Gamma-hydroxybutyrate accumulation in Arabidopsis and tobacco plants is a general response to abiotic stress: putative regulation by redox balance and glyoxylate reductase isoforms
Abstract
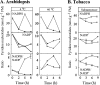
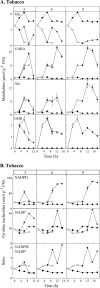
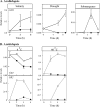
Enzymes that reduce the aldehyde chemical grouping (i.e. H-C=O) to its corresponding alcohol are probably crucial in maintaining plant health during stress. Succinic semialdehyde (SSA) is a mitochondrially-generated intermediate in the metabolism of gamma-aminobutyrate (GABA), which accumulates in response to a variety of biotic and abiotic stresses. SSA can be reduced to gamma-hydroxybutyrate (GHB) under oxygen deficiency and high light conditions. Recent evidence indicates that distinct cytosolic and plastidial glyoxylate reductase isoforms from Arabidopsis (designated herein after as AtGR1 and AtGR2, respectively) catalyse the in vitro conversion of SSA to GHB, as well as glyoxylate to glycolate, via NADPH-dependent reactions. In the present report, the responses of GHB and related amino acids, as well as NADP(+) and NADPH, were monitored in leaves from Arabidopsis or tobacco plants subjected to various abiotic stresses (i.e. Arabidopsis during exposure to salinity, drought, submergence, cold, or heat; tobacco during exposure to, and recovery from, submergence). Time-course experiments revealed that GHB accumulated in both Arabidopsis and tobacco plants subjected to stress, and that this accumulation was generally accompanied by higher GABA and alanine levels, higher NADPH/NADP(+) ratio, and lower glutamate levels. Furthermore, the analysis of gene expression in Arabidopsis revealed that the relative abundance of GR1 (salinity, drought, submergence, cold, and heat) and GR2 (cold and heat) transcripts was enhanced by the stress tested. Thus, GHB accumulation in plants is a general response to abiotic stress and appears to be regulated by both biochemical and transcriptional processes.
Figures

References
-
- Allan WL, Peiris C, Bown AW, Shelp BJ. Gamma-hydroxybutyrate accumulates in green tea leaves and soybean sprouts in response to oxygen deficiency. Canadian Journal of Plant Science. 2003a;83:951–953.
-
- Allan WL, Shelp BJ. Fluctuations of gamma-aminobutyrate, gamma-hydroxybutyrate and related amino acids in Arabidopsis leaves as a function of the light-dark cycle, leaf age, and N stress. Canadian Journal of Botany. 2006;84:1339–1346.
-
- Allan WL, Smith R, Shelp BJ. Application Bulletin AB-0015. Agilent Technologies Inc., Mississauga, ON,: Canada; 2003b. Direct measurement of γ-hydroxybutyrate (GHB) in crude plant extracts by liquid chromatography/negative ion-ES mass spectrometry; p. 4 pp.
-
- Bartels D. Targeting detoxification pathways: an efficient approach to obtain plants with multiple stress tolerance. Trends in Plant Science. 2001;6:284–286. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

