A high affinity hepatocyte growth factor-binding site in the immunoglobulin-like region of Met
- PMID: 18495663
- PMCID: PMC2475716
- DOI: 10.1074/jbc.M800727200
A high affinity hepatocyte growth factor-binding site in the immunoglobulin-like region of Met
Abstract
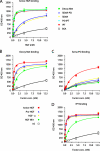
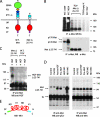
Hepatocyte growth factor (HGF) and its high affinity receptor, the tyrosine kinase Met, play a key role in embryo development and tumor invasion. Both HGF and Met are established targets for cancer therapy. However, the mechanism of their interaction is complex and remains elusive. HGF is secreted as a monomeric precursor (pro-HGF) that binds to but does not activate Met. Mature HGF is a alpha/beta heterodimer containing a high affinity Met-binding site in the alpha-chain (HGF-alpha) and a low affinity Met-binding site in the beta-chain (HGF-beta). The extracellular portion of Met contains a semaphorin (Sema) domain, a cysteine-rich hinge (plexin-semaphorin-integrin), and four immunoglobulin-like domains (immunoglobulin-like regions in plexins and transcription factors (IPT) 1-4). HGF-beta binds to Sema through a low affinity contact. The domain of Met responsible for high affinity binding to HGF-alpha has not been identified yet. Here we show that this long sought after binding site lies in the immunoglobulin-like region of Met and more precisely in IPT 3 and 4. We also show that IPT 3 and 4 are sufficient to transmit the signal for kinase activation to the cytoplasm, although the lack of Sema makes the receptor equally sensitive to mature HGF and pro-HGF. Finally, we provide evidence that soluble Met-derived proteins containing either the low affinity or high affinity HGF-binding site antagonize HGF-induced invasive growth both in vitro and in xenografts. These data suggest that the immunoglobulin-like region of Met cooperates with the Sema domain in binding to HGF and in controlling Met kinase activity. Although the IPT-HGF-alpha interaction provides binding strength, the Sema-HGF-beta contact confers selective sensitivity to the active form of the ligand.
Figures




References
-
- Bottaro, D. P., Rubin, J. S., Faletto, D. L., Chan, A. M., Kmiecik, T. E., Vande Woude, G. F., and Aaronson, S. A. (1991) Science 251 802-804 - PubMed
-
- Naldini, L., Vigna, E., Narsimhan, R. P., Gaudino, G., Zarnegar, R., Michalopoulos, G. K., and Comoglio, P. M. (1991) Oncogene 6 501-504 - PubMed
-
- Giordano, S., Ponzetto, C., Di Renzo, M. F., Cooper, C. S., and Comoglio, P. M. (1989) Nature 339 155-156 - PubMed
-
- Komada, M., Hatsuzawa, K., Shibamoto, S., Ito, F., Nakayama, K., and Kitamura, N. (1993) FEBS Lett. 328 25-29 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

