A single amino acid substitution in the murine norovirus capsid protein is sufficient for attenuation in vivo
- PMID: 18495770
- PMCID: PMC2493303
- DOI: 10.1128/JVI.00237-08
A single amino acid substitution in the murine norovirus capsid protein is sufficient for attenuation in vivo
Abstract
Murine norovirus (MNV), a prevalent pathogen of laboratory mice, shares many characteristics with human noroviruses. Previous results indicated that passage of MNV1 in the macrophage cell line RAW 264.7 results in attenuation in STAT1-deficient mice (C. E. Wobus, S. M. Karst, L. B. Thackray, K. O. Chang, S. V. Sosnovtsev, G. Belliot, A. Krug, J. M. Mackenzie, K. Y. Green, and H. W. Virgin, PLoS. Biol. 2:e432, 2004). Sequence analysis revealed two amino acid differences between the virulent and attenuated viruses. Using an infectious cDNA clone of the attenuated virus, we demonstrated that a glutamate-to-lysine substitution at position 296 in the capsid protein (VP1) is sufficient to restore virulence in vivo, identifying, for the first time, a virus-encoded molecular determinant of norovirus virulence.
Figures
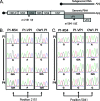
References
-
- Guo, M., J. Hayes, K. O. Cho, A. V. Parwani, L. M. Lucas, and L. J. Saif. 2001. Comparative pathogenesis of tissue culture-adapted and wild-type Cowden porcine enteric calicivirus (PEC) in gnotobiotic pigs and induction of diarrhea by intravenous inoculation of wild-type PEC. J. Virol. 759239-9251. - PMC - PubMed
-
- Hardy, M. E. 2005. Norovirus protein structure and function. FEMS Microbiol. Lett. 2531-8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

