Phosphorylation of UT-A1 urea transporter at serines 486 and 499 is important for vasopressin-regulated activity and membrane accumulation
- PMID: 18495802
- PMCID: PMC2494505
- DOI: 10.1152/ajprenal.00102.2008
Phosphorylation of UT-A1 urea transporter at serines 486 and 499 is important for vasopressin-regulated activity and membrane accumulation
Abstract
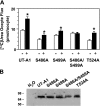
The UT-A1 urea transporter plays an important role in the urine concentrating mechanism. Vasopressin (or cAMP) increases urea permeability in perfused terminal inner medullary collecting ducts and increases the abundance of phosphorylated UT-A1, suggesting regulation by phosphorylation. We performed a phosphopeptide analysis that strongly suggested that a PKA consensus site(s) in the central loop region of UT-A1 was/were phosphorylated. Serine 486 was most strongly identified, with other potential sites at serine 499 and threonine 524. Phosphomutation constructs of each residue were made and transiently transfected into LLC-PK1 cells to assay for UT-A1 phosphorylation. The basal level of UT-A1 phosphorylation was unaltered by mutation of these sites. We injected oocytes, assayed [14C]urea flux, and determined that mutation of these sites did not alter basal urea transport activity. Next, we tested the effect of stimulating cAMP production with forskolin. Forskolin increased wild-type UT-A1 and T524A phosphorylation in LLC-PK1 cells and increased urea flux in oocytes. In contrast, the S486A and S499A mutants demonstrated loss of forskolin-stimulated UT-A1 phosphorylation and reduced urea flux. In LLC-PK1 cells, we assessed biotinylated UT-A1. Wild-type UT-A1, S486A, and S499A accumulated in the membrane in response to forskolin. However, in the S486A/S499A double mutant, forskolin-stimulated UT-A1 membrane accumulation and urea flux were totally blocked. We conclude that the phosphorylation of UT-A1 on both serines 486 and 499 is important for activity and that this phosphorylation may be involved in UT-A1 membrane accumulation.
Figures



References
-
- Blount MA, Klein JD, Martin CF, Tchapyjnikov D, Sands JM. Forskolin stimulates phosphorylation and membrane accumulation of UT-A3. Am J Physiol Renal Physiol 293: F1308–F1313, 2007. - PubMed
-
- Chabardes D, Firsov D, Aarab L, Clabecq A, Bellanger AC, Siaume-Perez S, Elalouf JM. Localization of mRNAs encoding Ca2+-inhibitable adenylyl cyclases along the renal tubule. Functional consequences for regulation of the cAMP content. J Biol Chem 271: 19264–19271, 1996. - PubMed
-
- Francis SH, Corbin JD. Cyclic nucleotide-dependent protein kinases: intracellular receptors for cAMP and cGMP action. Crit Rev Clin Lab Sci 36: 275–328, 1999. - PubMed
-
- Fröhlich O, Klein JD, Smith PM, Sands JM, Gunn RB. Regulation of UT-A1-mediated transepithelial urea flux in MDCK cells. Am J Physiol Cell Physiol 291: C600–C606, 2006. - PubMed
-
- Helies-Toussaint C, Aarab L, Gasc JM, Verbavatz JM, Chabardes D. Cellular localization of type 5 and type 6 ACs in collecting duct and regulation of cAMP synthesis. Am J Physiol Renal Physiol 279: F185–F194, 2000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

