Extracellular matrix-specific focal adhesions in vascular smooth muscle produce mechanically active adhesion sites
- PMID: 18495809
- PMCID: PMC2493553
- DOI: 10.1152/ajpcell.00516.2007
Extracellular matrix-specific focal adhesions in vascular smooth muscle produce mechanically active adhesion sites
Abstract
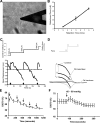
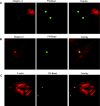
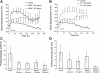
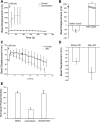
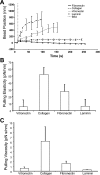
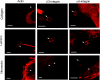
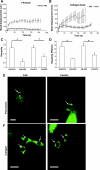
Integrin-mediated mechanotransduction in vascular smooth muscle cells (VSMCs) plays an important role in the physiological control of tissue blood flow and vascular resistance. To test whether force applied to specific extracellular matrix (ECM)-integrin interactions could induce myogenic-like mechanical activity at focal adhesion sites, we used atomic force microscopy (AFM) to apply controlled forces to specific ECM adhesion sites on arteriolar VSMCs. The tip of AFM probes were fused with a borosilicate bead (2 ~ 5 microm) coated with fibronectin (FN), collagen type I (CNI), laminin (LN), or vitronectin (VN). ECM-coated beads induced clustering of alpha(5)- and beta(3)-integrins and actin filaments at sites of bead-cell contact indicative of focal adhesion formation. Step increases of an upward (z-axis) pulling force (800 ~ 1,600 pN) applied to the bead-cell contact site for FN-specific focal adhesions induced a myogenic-like, force-generating response from the VSMC, resulting in a counteracting downward pull by the cell. This micromechanical event was blocked by cytochalasin D but was enhanced by jasplakinolide. Function-blocking antibodies to alpha(5)beta(1)- and alpha(v)beta(3)-integrins also blocked the micromechanical cell event in a concentration-dependent manner. Similar pulling experiments with CNI, VN, or LN failed to induce myogenic-like micromechanical events. Collectively, these results demonstrate that mechanical force applied to integrin-FN adhesion sites induces an actin-dependent, myogenic-like, micromechanical event. Focal adhesions formed by different ECM proteins exhibit different mechanical characteristics, and FN appears of particular relevance in its ability to strongly attach to VSMCs and to induce myogenic-like, force-generating reactions from sites of focal adhesion in response to externally applied forces.
Figures
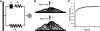
Similar articles
-
Mechanotransduction through fibronectin-integrin focal adhesion in microvascular smooth muscle cells: is calcium essential?Am J Physiol Heart Circ Physiol. 2012 May 15;302(10):H1965-73. doi: 10.1152/ajpheart.00598.2011. Epub 2012 Mar 16. Am J Physiol Heart Circ Physiol. 2012. PMID: 22427509 Free PMC article.
-
Mechanical properties of the interaction between fibronectin and alpha5beta1-integrin on vascular smooth muscle cells studied using atomic force microscopy.Am J Physiol Heart Circ Physiol. 2005 Dec;289(6):H2526-35. doi: 10.1152/ajpheart.00658.2004. Epub 2005 Aug 12. Am J Physiol Heart Circ Physiol. 2005. PMID: 16100245
-
Modulation of microvascular smooth muscle adhesion and mechanotransduction by integrin-linked kinase.Microcirculation. 2010 Feb;17(2):113-27. doi: 10.1111/j.1549-8719.2009.00011.x. Microcirculation. 2010. PMID: 20163538 Free PMC article.
-
Coordinated regulation of vascular Ca2+ and K+ channels by integrin signaling.Adv Exp Med Biol. 2010;674:69-79. doi: 10.1007/978-1-4419-6066-5_7. Adv Exp Med Biol. 2010. PMID: 20549941 Review.
-
Masters and servants of the force: the role of matrix adhesions in myofibroblast force perception and transmission.Eur J Cell Biol. 2006 Apr;85(3-4):175-81. doi: 10.1016/j.ejcb.2005.09.004. Epub 2005 Oct 10. Eur J Cell Biol. 2006. PMID: 16546559 Review.
Cited by
-
Zyxin is involved in regulation of mechanotransduction in arteriole smooth muscle cells.Front Physiol. 2012 Dec 20;3:472. doi: 10.3389/fphys.2012.00472. eCollection 2012. Front Physiol. 2012. PMID: 23267329 Free PMC article.
-
Cardiomyocyte contractile status is associated with differences in fibronectin and integrin interactions.Am J Physiol Heart Circ Physiol. 2010 Jun;298(6):H2071-81. doi: 10.1152/ajpheart.01156.2009. Epub 2010 Apr 9. Am J Physiol Heart Circ Physiol. 2010. PMID: 20382852 Free PMC article.
-
Nonenzymatic glycation interferes with fibronectin-integrin interactions in vascular smooth muscle cells.Microcirculation. 2017 Apr;24(3):10.1111/micc.12347. doi: 10.1111/micc.12347. Microcirculation. 2017. PMID: 28005306 Free PMC article.
-
N-Cadherin, a novel and rapidly remodelling site involved in vasoregulation of small cerebral arteries.J Physiol. 2017 Mar 15;595(6):1987-2000. doi: 10.1113/JP272995. Epub 2017 Feb 7. J Physiol. 2017. PMID: 28008617 Free PMC article.
-
Integrin α2β1 regulates collagen I tethering to modulate hyperresponsiveness in reactive airway disease models.J Clin Invest. 2021 Jun 15;131(12):e138140. doi: 10.1172/JCI138140. J Clin Invest. 2021. PMID: 33956668 Free PMC article.
References
-
- Alenghat FJ, Ingber DE. Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Sci STKE 2002: PE6, 2002. - PubMed
-
- Banes AJ, Tsuzaki M, Yamamoto J, Fischer T, Brigman B, Brown T, Miller L. Mechanoreception at the cellular level: the detection, interpretation, and diversity of responses to mechanical signals. Biochem Cell Biol 73: 349–365, 1995. - PubMed
-
- Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol 19: 677–695, 2003. - PubMed
-
- Calderwood DA, Fujioka Y, de Pereda JM, Garcia-Alvarez B, Nakamoto T, Margolis B, McGlade CJ, Liddington RC, Ginsberg MH. Integrin beta cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc Natl Acad Sci USA 100: 2272–2277, 2003. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

