Rho-family GTPases modulate Ca(2+) -dependent ATP release from astrocytes
- PMID: 18495810
- PMCID: PMC2493544
- DOI: 10.1152/ajpcell.00175.2008
Rho-family GTPases modulate Ca(2+) -dependent ATP release from astrocytes
Abstract
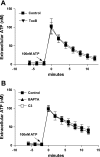
Previously, we reported that activation of G protein-coupled receptors (GPCR) in 1321N1 human astrocytoma cells elicits a rapid release of ATP that is partially dependent on a G(q)/phophospholipase C (PLC)/Ca(2+) mobilization signaling cascade. In this study we assessed the role of Rho-family GTPase signaling as an additional pathway for the regulation of ATP release in response to activation of protease-activated receptor-1 (PAR1), lysophosphatidic acid receptor (LPAR), and M3-muscarinic (M3R) GPCRs. Thrombin (or other PAR1 peptide agonists), LPA, and carbachol triggered quantitatively similar Ca(2+) mobilization responses, but only thrombin and LPA caused rapid accumulation of active GTP-bound Rho. The ability to elicit Rho activation correlated with the markedly higher efficacy of thrombin and LPA, relative to carbachol, as ATP secretagogues. Clostridium difficile toxin B and Clostridium botulinum C3 exoenzyme, which inhibit Rho-GTPases, attenuated the thrombin- and LPA-stimulated ATP release but did not decrease carbachol-stimulated release. Thus the ability of certain G(q)-coupled receptors to additionally stimulate Rho-GTPases acts to strongly potentiate a Ca(2+)-activated ATP release pathway. However, pharmacological inhibition of Rho kinase I/II or myosin light chain kinase did not attenuate ATP release. PAR1-induced ATP release was also reduced twofold by brefeldin treatment suggesting the possible mobilization of Golgi-derived, ATP-containing secretory vesicles. ATP release was also markedly repressed by the gap junction channel inhibitor carbenoxolone in the absence of any obvious thrombin-induced change in membrane permeability indicative of hemichannel gating.
Figures







Similar articles
-
Extracellular osmolarity modulates G protein-coupled receptor-dependent ATP release from 1321N1 astrocytoma cells.Am J Physiol Cell Physiol. 2010 Feb;298(2):C386-96. doi: 10.1152/ajpcell.00430.2009. Epub 2009 Nov 11. Am J Physiol Cell Physiol. 2010. PMID: 19907018 Free PMC article.
-
Galpha12/13- and rho-dependent activation of phospholipase C-epsilon by lysophosphatidic acid and thrombin receptors.Mol Pharmacol. 2006 Jun;69(6):2068-75. doi: 10.1124/mol.105.017921. Epub 2006 Mar 22. Mol Pharmacol. 2006. PMID: 16554409
-
Thrombin promotes release of ATP from lung epithelial cells through coordinated activation of rho- and Ca2+-dependent signaling pathways.J Biol Chem. 2009 Jul 31;284(31):20638-48. doi: 10.1074/jbc.M109.004762. Epub 2009 May 12. J Biol Chem. 2009. PMID: 19439413 Free PMC article.
-
[Analysis of synaptic neurotransmitter release mechanisms using bacterial toxins].J Soc Biol. 1999;193(6):457-67. J Soc Biol. 1999. PMID: 10783704 Review. French.
-
Clostridial ADP-ribosylating toxins: effects on ATP and GTP-binding proteins.Mol Cell Biochem. 1994 Sep;138(1-2):167-76. doi: 10.1007/BF00928459. Mol Cell Biochem. 1994. PMID: 7898461 Review.
Cited by
-
Signal pathways in astrocytes activated by cross-talk between of astrocytes and mast cells through CD40-CD40L.J Neuroinflammation. 2011 Mar 16;8:25. doi: 10.1186/1742-2094-8-25. J Neuroinflammation. 2011. PMID: 21410936 Free PMC article.
-
Lipoapoptosis induced by saturated free fatty acids stimulates monocyte migration: a novel role for Pannexin1 in liver cells.Purinergic Signal. 2015 Sep;11(3):347-59. doi: 10.1007/s11302-015-9456-5. Epub 2015 Jun 9. Purinergic Signal. 2015. PMID: 26054298 Free PMC article.
-
Histamine induces ATP release from human subcutaneous fibroblasts, via pannexin-1 hemichannels, leading to Ca2+ mobilization and cell proliferation.J Biol Chem. 2013 Sep 20;288(38):27571-27583. doi: 10.1074/jbc.M113.460865. Epub 2013 Aug 5. J Biol Chem. 2013. PMID: 23918924 Free PMC article. Clinical Trial.
-
Enteric Glial Cells: A New Frontier in Neurogastroenterology and Clinical Target for Inflammatory Bowel Diseases.Inflamm Bowel Dis. 2016 Feb;22(2):433-49. doi: 10.1097/MIB.0000000000000667. Inflamm Bowel Dis. 2016. PMID: 26689598 Free PMC article. Review.
-
Caffeine and the control of cerebral hemodynamics.J Alzheimers Dis. 2010;20 Suppl 1(Suppl 1):S51-62. doi: 10.3233/JAD-2010-091261. J Alzheimers Dis. 2010. PMID: 20182032 Free PMC article. Review.
References
-
- Abbracchio MP, Verderio C. Pathophysiological roles of P2 receptors in glial cells. Novartis Found Symp 276: 91–103, 2006. - PubMed
-
- Aktories K, Schmidt G, Just I. Rho GTPases as targets of bacterial protein toxins. Biol Chem 381: 421–426, 2000. - PubMed
-
- Aragay AM, Collins LR, Post GR, Watson AJ, Feramisco JR, Brown JH, Simon MI. G12 requirement for thrombin-stimulated gene expression and DNA synthesis in 1321N1 astrocytoma cells. J Biol Chem 270: 20073–20077, 1995. - PubMed
-
- Ashkenazi A, Peralta EG, Winslow JW, Ramachandran J, Capon DJ. Functional role of muscarinic acetylcholine receptor subtype diversity. Cold Spring Harb Symp Quant Biol 53: 263–272, 1988. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

