Antibodies to proteinase 3 prime human oral, lung, and kidney epithelial cells to secrete proinflammatory cytokines upon stimulation with agonists to various Toll-like receptors, NOD1, and NOD2
- PMID: 18495849
- PMCID: PMC2446640
- DOI: 10.1128/CVI.00137-08
Antibodies to proteinase 3 prime human oral, lung, and kidney epithelial cells to secrete proinflammatory cytokines upon stimulation with agonists to various Toll-like receptors, NOD1, and NOD2
Abstract
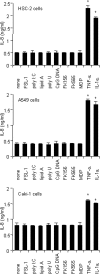
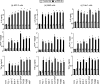
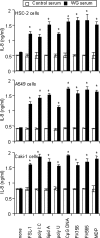
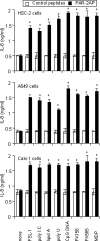
Antineutrophil cytoplasmic antibodies (ANCA) are autoantibodies, the detection of which in serum can be used in the diagnosis of Wegener's granulomatosis (WG). Proteinase 3 (PR3) is a major target antigen of ANCA in WG patients, and the interaction of PR3 ANCA with leukocytes causes a debilitating autoimmune disease. The first signs and symptoms in WG patients are observed in the oral cavity, lungs, and kidneys. Human epithelial cells generally do not secrete proinflammatory cytokines upon stimulation with pathogen-associated molecular patterns (PAMPs). In this study, anti-PR3 antibodies (Abs) and PR3 ANCA-containing sera from WG patients endowed human oral, lung, and kidney epithelial cells with responsiveness to PAMPs in terms of the production of proinflammatory cytokines, such as interleukin-6 (IL-6), IL-8, monocyte chemoattractant protein-1, and tumor necrosis factor alpha. Protease-activated receptor-2 (PAR-2) agonist peptides mimicked the priming effects of PR3 ANCA against PAMPs. Furthermore, the anti-PR3 Ab-mediated cell activation was significantly abolished by RNA interference targeting PAR-2 and NF-kappaB. This is the first report of priming effects of anti-PR3 Abs (PR3 ANCA) on epithelial cells. The results suggest that anti-PR3 Abs (PR3 ANCA) prime human epithelial cells to produce cytokines upon stimulation with various PAMPs, and these mechanisms may be involved in severe chronic inflammation in WG.
Figures

Similar articles
-
Antibodies to proteinase 3 prime human monocytic cells via protease-activated receptor-2 and NF-kappaB for Toll-like receptor- and NOD-dependent activation.Mol Immunol. 2007 Jul;44(14):3552-62. doi: 10.1016/j.molimm.2007.03.010. Epub 2007 Apr 23. Mol Immunol. 2007. PMID: 17452051
-
Proteinase 3, protease-activated receptor-2 and interleukin-32: linking innate and autoimmunity in Wegener's granulomatosis.Clin Exp Rheumatol. 2008 May-Jun;26(3 Suppl 49):S112-7. Clin Exp Rheumatol. 2008. PMID: 18799068 Review.
-
Proinflammatory cytokines induce proteinase 3 as membrane-bound and secretory forms in human oral epithelial cells and antibodies to proteinase 3 activate the cells through protease-activated receptor-2.J Immunol. 2004 Sep 15;173(6):4179-89. doi: 10.4049/jimmunol.173.6.4179. J Immunol. 2004. Retraction in: J Immunol. 2010 Apr 1;184(7):4044. doi: 10.4049/jimmunol.1090013. PMID: 15356169 Retracted.
-
Synergism between TLRs and NOD1/2 in oral epithelial cells.J Dent Res. 2008 Jul;87(7):682-6. doi: 10.1177/154405910808700709. J Dent Res. 2008. PMID: 18573991
-
Pathogenicity of Proteinase 3-Anti-Neutrophil Cytoplasmic Antibody in Granulomatosis With Polyangiitis: Implications as Biomarker and Future Therapies.Front Immunol. 2021 Feb 18;12:571933. doi: 10.3389/fimmu.2021.571933. eCollection 2021. Front Immunol. 2021. PMID: 33679731 Free PMC article. Review.
Cited by
-
Evaluation of Wegener's granulomatosis using 18F-fluorodeoxyglucose positron emission tomography/computed tomography.Ann Nucl Med. 2013 Apr;27(3):209-16. doi: 10.1007/s12149-012-0675-3. Epub 2012 Dec 16. Ann Nucl Med. 2013. PMID: 23242952 Free PMC article.
-
Novel signaling interactions between proteinase-activated receptor 2 and Toll-like receptors in vitro and in vivo.Mucosal Immunol. 2010 Jan;3(1):29-39. doi: 10.1038/mi.2009.120. Epub 2009 Oct 28. Mucosal Immunol. 2010. PMID: 19865078 Free PMC article.
-
NOD-like receptors and inflammation.Arthritis Res Ther. 2008;10(6):228. doi: 10.1186/ar2525. Epub 2008 Nov 25. Arthritis Res Ther. 2008. PMID: 19090963 Free PMC article. Review.
-
Cross-talk between toll-like receptor 4 (TLR4) and proteinase-activated receptor 2 (PAR(2) ) is involved in vascular function.Br J Pharmacol. 2013 Jan;168(2):411-20. doi: 10.1111/j.1476-5381.2012.02205.x. Br J Pharmacol. 2013. PMID: 22957757 Free PMC article.
-
Update on pathogenic mechanisms of systemic necrotizing vasculitis.Curr Rheumatol Rep. 2008 Dec;10(6):430-5. doi: 10.1007/s11926-008-0070-1. Curr Rheumatol Rep. 2008. PMID: 19007531 Review.
References
-
- Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. - PubMed
-
- Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. - PubMed
-
- Bartůn̆ková, J., V. Tesař, and A. Šedivá. 2003. Diagnostic and pathogenetic role of antineutrophil cytoplasmic autoantibodies. Clin. Immunol. 106: 73-82. - PubMed
-
- Casselman, B. L., K. S. Kilgore, B. F. Miller, and J. S. Warren. 1995. Antibodies to neutrophil cytoplasmic antigens induce monocyte chemoattractant protein-1 secretion from human monocytes. J. Lab. Clin. Med. 126:495-502. - PubMed
-
- Chamaillard, M., M. Hashimoto, Y. Horie, J. Masumoto, S. Qiu, L. Saab, Y. Ogura, A. Kawasaki, K. Fukase, S. Kusumoto, M. A. Valvano, S. J. Foster, T. W. Mak, G. Nuñez, and N. Inohara. 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4:702-707. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

