Antibodies to proteinase 3 prime human oral, lung, and kidney epithelial cells to secrete proinflammatory cytokines upon stimulation with agonists to various Toll-like receptors, NOD1, and NOD2
- PMID: 18495849
- PMCID: PMC2446640
- DOI: 10.1128/CVI.00137-08
Antibodies to proteinase 3 prime human oral, lung, and kidney epithelial cells to secrete proinflammatory cytokines upon stimulation with agonists to various Toll-like receptors, NOD1, and NOD2
Abstract
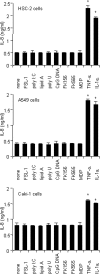
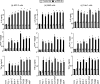
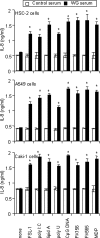
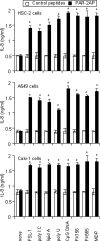
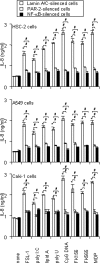
Antineutrophil cytoplasmic antibodies (ANCA) are autoantibodies, the detection of which in serum can be used in the diagnosis of Wegener's granulomatosis (WG). Proteinase 3 (PR3) is a major target antigen of ANCA in WG patients, and the interaction of PR3 ANCA with leukocytes causes a debilitating autoimmune disease. The first signs and symptoms in WG patients are observed in the oral cavity, lungs, and kidneys. Human epithelial cells generally do not secrete proinflammatory cytokines upon stimulation with pathogen-associated molecular patterns (PAMPs). In this study, anti-PR3 antibodies (Abs) and PR3 ANCA-containing sera from WG patients endowed human oral, lung, and kidney epithelial cells with responsiveness to PAMPs in terms of the production of proinflammatory cytokines, such as interleukin-6 (IL-6), IL-8, monocyte chemoattractant protein-1, and tumor necrosis factor alpha. Protease-activated receptor-2 (PAR-2) agonist peptides mimicked the priming effects of PR3 ANCA against PAMPs. Furthermore, the anti-PR3 Ab-mediated cell activation was significantly abolished by RNA interference targeting PAR-2 and NF-kappaB. This is the first report of priming effects of anti-PR3 Abs (PR3 ANCA) on epithelial cells. The results suggest that anti-PR3 Abs (PR3 ANCA) prime human epithelial cells to produce cytokines upon stimulation with various PAMPs, and these mechanisms may be involved in severe chronic inflammation in WG.
Figures

References
-
- Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. - PubMed
-
- Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. - PubMed
-
- Bartůn̆ková, J., V. Tesař, and A. Šedivá. 2003. Diagnostic and pathogenetic role of antineutrophil cytoplasmic autoantibodies. Clin. Immunol. 106: 73-82. - PubMed
-
- Casselman, B. L., K. S. Kilgore, B. F. Miller, and J. S. Warren. 1995. Antibodies to neutrophil cytoplasmic antigens induce monocyte chemoattractant protein-1 secretion from human monocytes. J. Lab. Clin. Med. 126:495-502. - PubMed
-
- Chamaillard, M., M. Hashimoto, Y. Horie, J. Masumoto, S. Qiu, L. Saab, Y. Ogura, A. Kawasaki, K. Fukase, S. Kusumoto, M. A. Valvano, S. J. Foster, T. W. Mak, G. Nuñez, and N. Inohara. 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4:702-707. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

