Human coronavirus NL63 and 229E seroconversion in children
- PMID: 18495857
- PMCID: PMC2446899
- DOI: 10.1128/JCM.00533-08
Human coronavirus NL63 and 229E seroconversion in children
Abstract
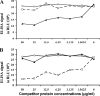
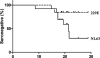
In 2004, the novel respiratory human coronavirus NL63 (HCoV-NL63) was identified, and subsequent research revealed that the virus has spread worldwide. HCoV-229E is a close relative of HCoV-NL63, and infection with either virus can lead to the hospitalization of young children, immunocompromised persons, and the elderly. Children infected with HCoV-NL63 often develop croup, with obstruction of the airway. In this study we investigated at which age children are confronted for the first time with an HCoV-NL63 infection and, thus, at which age they seroconvert to HCoV-NL63 positivity. We designed a recombinant HCoV-229E and a recombinant HCoV-NL63 nucleocapsid protein enzyme-linked immunosorbent assay and performed a seroepidemiology survey on longitudinal and cross-sectional serum samples. The longitudinal serum samples were collected from 13 newborns, and data for those newborns were available from multiple time points spanning a period of at least 18 months. For the cross-sectional survey we tested serum samples of 139 children, including newborns to children 16 years of age. In examinations of the longitudinal serum samples we observed that all of the children had maternal anti-NL63 and anti-229E antibodies at birth that disappeared within 3 months. Seven of the 13 children became HCoV-NL63 seropositive during follow-up, whereas only 2 became HCoV-229E seropositive. The serology data of the cross-sectional serum samples revealed that 75% and 65% of the children in the age group 2.5 to 3.5 years were HCoV-NL63 and HCoV-229E seropositive, respectively. We conclude that on average, HCoV-NL63 and HCoV-229E seroconversion occurs before children reach the age of 3.5 years.
Figures

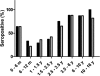
References
-
- Chibo, D., and C. Birch. 2006. Analysis of human coronavirus 229E spike and nucleoprotein genes demonstrates genetic drift between chronologically distinct strains. J. Gen. Virol. 871203-1208. - PubMed
-
- Drosten, C., S. Gunther, W. Preiser, S. van der Werf, H. R. Brodt, S. Becker, H. Rabenau, M. Panning, L. Kolesnikova, R. A. Fouchier, A. Berger, A. M. Burguiere, J. Cinatl, M. Eickmann, N. Escriou, K. Grywna, S. Kramme, J. C. Manuguerra, S. Muller, V. Rickerts, M. Sturmer, S. Vieth, H. D. Klenk, A. D. Osterhaus, H. Schmitz, and H. W. Doerr. 2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 3481967-1976. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

