The role of myosin II in glioma invasion of the brain
- PMID: 18495866
- PMCID: PMC2488307
- DOI: 10.1091/mbc.e08-03-0319
The role of myosin II in glioma invasion of the brain
Abstract
The ability of gliomas to invade the brain limits the efficacy of standard therapies. In this study, we have examined glioma migration in living brain tissue by using two novel in vivo model systems. Within the brain, glioma cells migrate like nontransformed, neural progenitor cells-extending a prominent leading cytoplasmic process followed by a burst of forward movement by the cell body that requires myosin II. In contrast, on a two-dimensional surface, glioma cells migrate more like fibroblasts, and they do not require myosin II to move. To explain this phenomenon, we studied glioma migration through a series of synthetic membranes with defined pore sizes. Our results demonstrate that the A and B isoforms of myosin II are specifically required when a glioma cell has to squeeze through pores smaller than its nuclear diameter. They support a model in which the neural progenitor-like mode of glioma invasion and the requirement for myosin II represent an adaptation needed to move within the brain, which has a submicrometer effective pore size. Furthermore, the absolute requirement for myosin II in brain invasion underscores the importance of this molecular motor as a potential target for new anti-invasive therapies to treat malignant brain tumors.
Figures
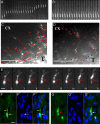

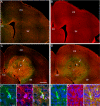
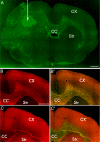
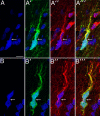

Comment in
- Mol Biol Cell. 19:3179.
References
-
- Amano M., Ito M., Kimura K., Fukata Y., Chihara K., Nakano T., Matsuura Y., Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J. Biol. Chem. 1996;271:20246–20249. - PubMed
-
- Bellion A., Baudoin J. P., Alvarez C., Bornens M., Metin C. Nucleokinesis in tangentially migrating neurons comprises two alternating phases: forward migration of the Golgi/centrosome associated with centrosome splitting and myosin contraction at the rear. J. Neurosci. 2005;25:5691–5699. - PMC - PubMed
-
- Betapudi V., Licate L. S., Egelhoff T. T. Distinct roles of nonmuscle myosin II isoforms in the regulation of MDA-MB-231 breast cancer cell spreading and migration. Cancer Res. 2006;66:4725–4733. - PubMed
-
- Bruehlmeier M., Roelcke U., Blauenstein P., Missimer J., Schubiger P. A., Locher J. T., Pellikka R., Ametamey S. M. Measurement of the extracellular space in brain tumors using 76Br-bromide and PET. J. Nucl. Med. 2003;44:1210–1218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

