More than a repair enzyme: Aspergillus nidulans photolyase-like CryA is a regulator of sexual development
- PMID: 18495868
- PMCID: PMC2488289
- DOI: 10.1091/mbc.e08-01-0061
More than a repair enzyme: Aspergillus nidulans photolyase-like CryA is a regulator of sexual development
Abstract
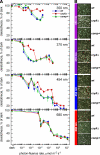
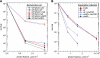
Cryptochromes are blue-light receptors that have presumably evolved from the DNA photolyase protein family, and the genomes of many organisms contain genes for both types of molecules. Both protein structures resemble each other, which suggests that light control and light protection share a common ancient origin. In the genome of the filamentous fungus Aspergillus nidulans, however, only one cryptochrome/photolyase-encoding gene, termed cryA, was identified. Deletion of the cryA gene triggers sexual differentiation under inappropriate culture conditions and results in up-regulation of transcripts encoding regulators of fruiting body formation. CryA is a protein whose N- and C-terminal synthetic green fluorescent protein fusions localize to the nucleus. CryA represses sexual development under UVA (350-370 nm) light both on plates and in submerged culture. Strikingly, CryA exhibits photorepair activity as demonstrated by heterologous complementation of a DNA repair-deficient Escherichia coli strain as well as overexpression in an A. nidulans uvsBDelta genetic background. This is in contrast to the single deletion cryADelta strain, which does not show increased sensitivity toward UV-induced damage. In A. nidulans, cryA encodes a novel type of cryptochrome/photolyase that exhibits a regulatory function during light-dependent development and DNA repair activity. This represents a paradigm for the evolutionary transition between photolyases and cryptochromes.
Figures



References
-
- Bayram O., Krappmann S., Seiler S., Vogt N., Braus G. H. Neurospora crassa ve-1 affects asexual conidiation. Fungal Genet. Biol. 2008;45:127–138. - PubMed
-
- Blumenstein A., Vienken K., Tasler R., Purschwitz J., Veith D., Frankenberg-Dinkel N., Fischer R. The Aspergillus nidulans phytochrome FphA represses sexual development in red light. Curr. Biol. 2005;15:1833–1838. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

