Entropic contributions and the influence of the hydrophobic environment in promiscuous protein-protein association
- PMID: 18495919
- PMCID: PMC2391134
- DOI: 10.1073/pnas.0800452105
Entropic contributions and the influence of the hydrophobic environment in promiscuous protein-protein association
Abstract
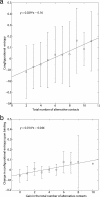
The mechanisms by which a promiscuous protein can strongly interact with several different proteins using the same binding interface are not completely understood. An example is protein kinase A (PKA), which uses a single face on its docking/dimerization domain to interact with multiple A-kinase anchoring proteins (AKAP) that localize it to different parts of the cell. In the current study, the configurational entropy contributions to the binding between the AKAP protein HT31 with the D/D domain of RII alpha-regulatory subunit of PKA were examined. The results show that the majority of configurational entropy loss for the interaction was due to decreased fluctuations within rotamer states of the side chains. The result is in contrast to the widely held approximation that the decrease in the number of rotamer states available to the side chains forms the major component. Further analysis showed that there was a direct linear relationship between total configurational entropy and the number of favorable, alternative contacts available within hydrophobic environments. The hydrophobic binding pocket of the D/D domain provides alternative contact points for the side chains of AKAP peptides that allow them to adopt different binding conformations. The increase in binding conformations provides an increase in binding entropy and hence binding affinity. We infer that a general strategy for a promiscuous protein is to provide alternative contact points at its interface to increase binding affinity while the plasticity required for binding to multiple partners is retained. Implications are discussed for understanding and treating diseases in which promiscuous protein interactions are used.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Alberts B. Molecular Biology of the Cell. New York: Garland Science; 2002.
-
- Buck E, Iyengar R. Organization and functions of interacting domains for signaling by protein–protein interactions. Sci STKE. 2003;2003:re14. - PubMed
-
- Colledge M, Scott JD. AKAPs: From structure to function. Trends Cell Biol. 1999;9:216–221. - PubMed
-
- Kim WK, Ison JC. Survey of the geometric association of domain–domain interfaces. Proteins. 2005;61:1075–1088. - PubMed
-
- Chow MK, Lomas DA, Bottomley SP. Promiscuous beta-strand interactions and the conformational diseases. Curr Med Chem. 2004;11:491–499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

