Complementary dimerization of microtubule-associated tau protein: Implications for microtubule bundling and tau-mediated pathogenesis
- PMID: 18495933
- PMCID: PMC2396711
- DOI: 10.1073/pnas.0802036105
Complementary dimerization of microtubule-associated tau protein: Implications for microtubule bundling and tau-mediated pathogenesis
Abstract
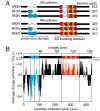
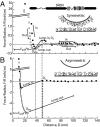
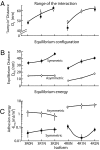
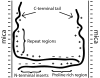
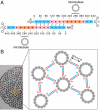
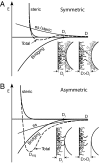
Tau is an intrinsically unstructured microtubule (MT)-associated protein capable of binding to and organizing MTs into evenly spaced parallel assemblies known as "MT bundles." How tau achieves MT bundling is enigmatic because each tau molecule possesses only one MT-binding region. To dissect this complex behavior, we have used a surface forces apparatus to measure the interaction forces of the six CNS tau isoforms when bound to mica substrates in vitro. Two types of measurements were performed for each isoform: symmetric configuration experiments measured the interactions between two tau-coated mica surfaces, whereas "asymmetric" experiments examined tau-coated surfaces interacting with a smooth bare mica surface. Depending on the configuration (of which there were 12), the forces were weakly adhesive, strongly adhesive, or purely repulsive. The equilibrium spacing was determined mainly by the length of the tau projection domain, in contrast to the adhesion force/energy, which was determined by the number of repeats in the MT-binding region. Taken together, the data are incompatible with tau acting as a monomer; rather, they indicate that two tau molecules associate in an antiparallel configuration held together by an electrostatic "zipper" of complementary salt bridges composed of the N-terminal and central regions of each tau monomer, with the C-terminal MT-binding regions extending outward from each end of the dimeric backbone. This tau dimer determines the length and strength of the linker holding two MTs together and could be the fundamental structural unit of tau, underlying both its normal and pathological action.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Chapin S, Bulinski J, Gundersen G. Microtubule bundling in cells. Nature. 1991;349:24. - PubMed
-
- Chen J, Kanai Y, Cowan N, Hirokawa N. Projection domains of Map2 and tau determine spacings between microtubules in dendrites and axons. Nature. 1992;360:674–676. - PubMed
-
- Scott CW, et al. Tau protein induces bundling of microtubules in vitro—Comparison of different tau isoforms and a tau protein-fragment. J Neurosci Res. 1992;33:19–29. - PubMed
-
- Brandt R, Lee G. Functional-organization of microtubule-associated protein-tau—Identification of regions which affect microtubule growth, nucleation, and bundle formation in vitro. J Biol Chem. 1993;268:3414–3419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

