A phosphatase cascade by which rewarding stimuli control nucleosomal response
- PMID: 18496528
- PMCID: PMC2796210
- DOI: 10.1038/nature06994
A phosphatase cascade by which rewarding stimuli control nucleosomal response
Abstract
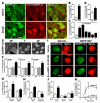
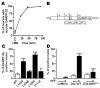
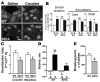
Dopamine orchestrates motor behaviour and reward-driven learning. Perturbations of dopamine signalling have been implicated in several neurological and psychiatric disorders, and in drug addiction. The actions of dopamine are mediated in part by the regulation of gene expression in the striatum, through mechanisms that are not fully understood. Here we show that drugs of abuse, as well as food reinforcement learning, promote the nuclear accumulation of 32-kDa dopamine-regulated and cyclic-AMP-regulated phosphoprotein (DARPP-32). This accumulation is mediated through a signalling cascade involving dopamine D1 receptors, cAMP-dependent activation of protein phosphatase-2A, dephosphorylation of DARPP-32 at Ser 97 and inhibition of its nuclear export. The nuclear accumulation of DARPP-32, a potent inhibitor of protein phosphatase-1, increases the phosphorylation of histone H3, an important component of nucleosomal response. Mutation of Ser 97 profoundly alters behavioural effects of drugs of abuse and decreases motivation for food, underlining the functional importance of this signalling cascade.
Figures


References
-
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. - PubMed
-
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. - PubMed
-
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol. 1999;375:13–30. - PubMed
-
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. - PubMed
-
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

