Bcl-2 family members: dual regulators of apoptosis and autophagy
- PMID: 18497563
- PMCID: PMC2749577
- DOI: 10.4161/auto.6260
Bcl-2 family members: dual regulators of apoptosis and autophagy
Abstract
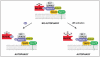
The essential autophagy protein and haplo-insufficient tumor suppressor, Beclin 1, interacts with several cofactors (Ambra1, Bif-1, UVRAG) to activate the lipid kinase Vps34, thereby inducing autophagy. In normal conditions, Beclin 1 is bound to and inhibited by Bcl-2 or the Bcl-2 homolog Bcl-X(L). This interaction involves a Bcl-2 homology 3 (BH3) domain in Beclin 1 and the BH3 binding groove of Bcl-2/Bcl-X(L). Other proteins containing BH3 domains, called BH3-only proteins, can competitively disrupt the interaction between Beclin 1 and Bcl-2/Bcl-X(L) to induce autophagy. Nutrient starvation, which is a potent physiologic inducer of autophagy, can stimulate the dissociation of Beclin 1 from its inhibitors, either by activating BH3-only proteins (such as Bad) or by posttranslational modifications of Bcl-2 (such as phosphorylation) that may reduce its affinity for Beclin 1 and BH3-only proteins. Thus, anti-apoptotic Bcl-2 family members and pro-apoptotic BH3-only proteins may participate in the inhibition and induction of autophagy, respectively. This hitherto neglected crosstalk between the core machineries regulating autophagy and apoptosis may redefine the role of Bcl-2 family proteins in oncogenesis and tumor progression.
Figures
References
-
- Kroemer G, El-Deiry WS, Golstein P, Peter ME, Vaux D, Vandenabeele P, Zhivotovsky B, Blagosklonny MV, Malorni W, Knight RA, Piacentini M, Nagata S, Melino G. Nomenclature Committee of Cell Death. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005;12:1463–7. - PubMed
-
- Galluzzi L, Maiuri MC, Vitale I, Zischka H, Castedo M, Zotvogel L, Kroemer G. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. 2007;14:1237–43. - PubMed
-
- Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–7. - PubMed
-
- Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439–48. - PubMed
-
- Maiuri C, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
