Molecular mechanism and structure of Trigger Factor bound to the translating ribosome
- PMID: 18497744
- PMCID: PMC2426727
- DOI: 10.1038/emboj.2008.89
Molecular mechanism and structure of Trigger Factor bound to the translating ribosome
Abstract
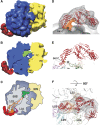
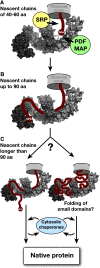
Ribosome-associated chaperone Trigger Factor (TF) initiates folding of newly synthesized proteins in bacteria. Here, we pinpoint by site-specific crosslinking the sequence of molecular interactions of Escherichia coli TF and nascent chains during translation. Furthermore, we provide the first full-length structure of TF associated with ribosome-nascent chain complexes by using cryo-electron microscopy. In its active state, TF arches over the ribosomal exit tunnel accepting nascent chains in a protective void. The growing nascent chain initially follows a predefined path through the entire interior of TF in an unfolded conformation, and even after folding into a domain it remains accommodated inside the protective cavity of ribosome-bound TF. The adaptability to accept nascent chains of different length and folding states may explain how TF is able to assist co-translational folding of all kinds of nascent polypeptides during ongoing synthesis. Moreover, we suggest a model of how TF's chaperoning function can be coordinated with the co-translational processing and membrane targeting of nascent polypeptides by other ribosome-associated factors.
Figures



References
-
- Agashe VR, Guha S, Chang HC, Genevaux P, Hayer-Hartl M, Stemp M, Georgopoulos C, Hartl FU, Barral JM (2004) Function of trigger factor and DnaK in multidomain protein folding: increase in yield at the expense of folding speed. Cell 117: 199–209 - PubMed
-
- Ball LA, Kaesberg P (1973) Cleavage of the N-terminal formylmethionine residue from a bacteriophage coat protein in vitro. J Mol Biol 79: 531–537 - PubMed
-
- Blanco FJ, Angrand I, Serrano L (1999) Exploring the conformational properties of the sequence space between two proteins with different folds: an experimental study. J Mol Biol 285: 741–753 - PubMed
-
- Bukau B, Deuerling E, Pfund C, Craig EA (2000) Getting newly synthesized proteins into shape. Cell 101: 119–122 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

