Ubiquitin docking at the proteasome through a novel pleckstrin-homology domain interaction
- PMID: 18497827
- PMCID: PMC2825158
- DOI: 10.1038/nature06924
Ubiquitin docking at the proteasome through a novel pleckstrin-homology domain interaction
Abstract
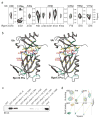
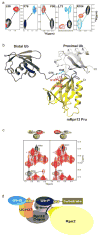
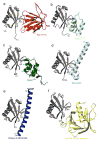
Targeted protein degradation is largely performed by the ubiquitin-proteasome pathway, in which substrate proteins are marked by covalently attached ubiquitin chains that mediate recognition by the proteasome. It is currently unclear how the proteasome recognizes its substrates, as the only established ubiquitin receptor intrinsic to the proteasome is Rpn10/S5a (ref. 1), which is not essential for ubiquitin-mediated protein degradation in budding yeast. In the accompanying manuscript we report that Rpn13 (refs 3-7), a component of the nine-subunit proteasome base, functions as a ubiquitin receptor, complementing its known role in docking de-ubiquitinating enzyme Uch37/UCHL5 (refs 4-6) to the proteasome. Here we merge crystallography and NMR data to describe the ubiquitin-binding mechanism of Rpn13. We determine the structure of Rpn13 alone and complexed with ubiquitin. The co-complex reveals a novel ubiquitin-binding mode in which loops rather than secondary structural elements are used to capture ubiquitin. Further support for the role of Rpn13 as a proteasomal ubiquitin receptor is demonstrated by its ability to bind ubiquitin and proteasome subunit Rpn2/S1 simultaneously. Finally, we provide a model structure of Rpn13 complexed to diubiquitin, which provides insights into how Rpn13 as a ubiquitin receptor is coupled to substrate deubiquitination by Uch37.
Figures
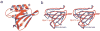
Comment in
-
Cell biology: two hands for degradation.Nature. 2008 May 22;453(7194):460-1. doi: 10.1038/453460a. Nature. 2008. PMID: 18497808 No abstract available.
References
-
- Deveraux Q, Ustrell V, Pickart C, Rechsteiner M. A 26 S protease subunit that binds ubiquitin conjugates. J Biol Chem. 1994;269:7059–61. - PubMed
-
- Fu H, et al. Multiubiquitin chain binding and protein degradation are mediated by distinct domains within the 26 S proteasome subunit Mcb1. J Biol Chem. 1998;273:1970–81. - PubMed
-
- Jorgensen JP, et al. Adrm1, a putative cell adhesion regulating protein, is a novel proteasome-associated factor. J Mol Biol. 2006;360:1043–52. - PubMed
-
- Yao T, et al. Proteasome recruitment and activation of the Uch37 deubiquitinating enzyme by Adrm1. Nat Cell Biol. 2006;8:994–1002. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

