Role for A-type lamins in herpesviral DNA targeting and heterochromatin modulation
- PMID: 18497856
- PMCID: PMC2374905
- DOI: 10.1371/journal.ppat.1000071
Role for A-type lamins in herpesviral DNA targeting and heterochromatin modulation
Abstract
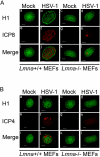
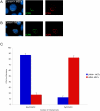
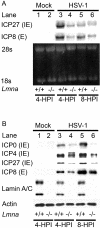
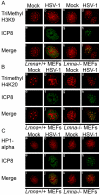
Posttranslational modification of histones is known to regulate chromatin structure and transcriptional activity, and the nuclear lamina is thought to serve as a site for heterochromatin maintenance and transcriptional silencing. In this report, we show that the nuclear lamina can also play a role in the downregulation of heterochromatin and in gene activation. Herpes simplex virus DNA initiates replication in replication compartments near the inner edge of the nucleus, and histones are excluded from these structures. To define the role of nuclear lamins in HSV replication, we examined HSV infection in wild-type and A-type lamin-deficient (Lmna-/-) murine embryonic fibroblasts (MEFs). In Lmna-/- cells, viral replication compartments are reduced in size and fail to target to the nuclear periphery, as observed in WT cells. Chromatin immunoprecipitation and immunofluorescence studies demonstrate that HSV DNA is associated with increased heterochromatin in Lmna-/- MEFs. These results argue for a functional role for A-type lamins as viral gene expression, DNA replication, and growth are reduced in Lmna-/- MEFs, with the greatest effect on viral replication at low multiplicity of infection. Thus, lamin A/C is required for targeting of the viral genome and the reduction of heterochromatin on viral promoters during lytic infection. The nuclear lamina can serve as a molecular scaffold for DNA genomes and the protein complexes that regulate both euchromatin and heterochromatin histone modifications.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

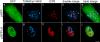
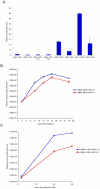
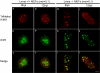
References
-
- Roizman B, Knipe DM, Whitley RJ. Herpes Simplex Virus. In: Knipe DM, Howley PM, editors. Fields Virology. 5th ed. Philadelphia: Lippincott, Williams and Wilkins; 2007. pp. 2501–2602.
-
- Quinlan MP, Chen LB, Knipe DM. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell. 1984;36:857–868. - PubMed
-
- de Bruyn Kops A, Knipe DM. Formation of DNA replication structures in herpes virus-infected cells requires a viral DNA binding protein. Cell. 1988;55:857–868. - PubMed
-
- Taylor TJ. Intranuclear localization of the herpes simplex virus ICP8 protein [Dissertation] Cambridge, MA: Harvard University; 2002.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

