HIV protease inhibitors provide neuroprotection through inhibition of mitochondrial apoptosis in mice
- PMID: 18497877
- PMCID: PMC2391064
- DOI: 10.1172/JCI34267
HIV protease inhibitors provide neuroprotection through inhibition of mitochondrial apoptosis in mice
Abstract
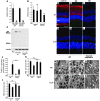
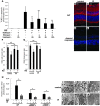
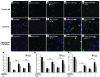
Neuroprotection can be achieved by preventing apoptotic death of postmitotic cells. Apoptotic death can occur by either a caspase-dependent mechanism, involving cytochrome c, apoptosis protease-activating factor-1 (Apaf-1), and caspase-9, or a caspase-independent mechanism, involving apoptosis-inducing factor (AIF). HIV protease inhibitors (PIs) avert apoptosis in part by preventing mitochondrial outer membrane permeabilization (MOMP), but the precise mechanism by which they work is not known. Here, we evaluated the impact of the PIs in a mouse model of retinal detachment (RD) in vivo and in murine primary retinal cell cultures in vitro. Oral administration of the PIs nelfinavir and ritonavir significantly inhibited photoreceptor apoptosis, while preventing the translocation of AIF from mitochondria to the nucleus as well as the activation of caspase-9. RD-induced photoreceptor apoptosis was similarly inhibited in mice carrying hypomorphic mutations of the genes encoding AIF or Apaf-1. Nelfinavir attenuated apoptosis as well as mitochondrial release of AIF and cytochrome c, and subsequent activation of caspase-9 in vitro, in photoreceptor cultures exposed to starvation or monocyte chemoattractant protein-1-stimulated (MCP-1-stimulated) macrophages. Our results suggest that the MOMP inhibition by PIs involved interruption of both caspase-dependent and caspase-independent apoptosis pathways and that PIs may be clinically useful for the treatment of diseases caused by excessive apoptosis.
Figures






References
-
- Dunaief J.L., Dentchev T., Ying G.S., Milam A.H. The role of apoptosis in age-related macular degeneration. Arch. Ophthalmol. 2002;120:1435–1442. - PubMed
-
- Fulton A.B., Hansen R.M., Petersen R.A., Vanderveen D.K. The rod photoreceptors in retinopathy of prematurity: an electroretinographic study. Arch. Ophthalmol. 2001;119:499–505. - PubMed
-
- Cook B., Lewis G.P., Fisher S.K., Adler R. Apoptotic photoreceptor degeneration in experimental retinal detachment. Invest. Ophthalmol. Vis. Sci. 1995;36:990–996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

