Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis
- PMID: 18497880
- PMCID: PMC2391067
- DOI: 10.1172/JCI34610
Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis
Abstract
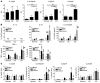
Intestinal macrophages play a central role in regulation of immune responses against commensal bacteria. In general, intestinal macrophages lack the expression of innate-immune receptor CD14 and do not produce proinflammatory cytokines against commensal bacteria. In this study, we identified what we believe to be a unique macrophage subset in human intestine. This subset expressed both macrophage (CD14, CD33, CD68) and DC markers (CD205, CD209) and produced larger amounts of proinflammatory cytokines, such as IL-23, TNF-alpha, and IL-6, than typical intestinal resident macrophages (CD14-CD33+ macrophages). In patients with Crohn disease (CD), the number of these CD14+ macrophages were significantly increased compared with normal control subjects. In addition to increased numbers of cells, these cells also produced larger amounts of IL-23 and TNF-alpha compared with those in normal controls or patients with ulcerative colitis. In addition, the CD14+ macrophages contributed to IFN-gamma production rather than IL-17 production by lamina propria mononuclear cells (LPMCs) dependent on IL-23 and TNF-alpha. Furthermore, the IFN-gamma produced by LPMCs triggered further abnormal macrophage differentiation with an IL-23-hyperproducing phenotype. Collectively, these data suggest that this IL-23/IFN-gamma-positive feedback loop induced by abnormal intestinal macrophages contributes to the pathogenesis of chronic intestinal inflammation in patients with CD.
Figures





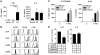
References
-
- Fuss I.J., et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J. Immunol. 1996;157:1261–1270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

