Detection of epithelial-cell injury, and quantification of infection, in the HCT-8 organoid model of cryptosporidiosis
- PMID: 18498239
- PMCID: PMC2631281
- DOI: 10.1086/588819
Detection of epithelial-cell injury, and quantification of infection, in the HCT-8 organoid model of cryptosporidiosis
Abstract
Background: Intestinal cells grown in microgravity produce a three-dimensional tissue assembly, or "organoid," similar to the human intestinal mucosa, making it an ideal model for enteric infections such as cryptosporidiosis.
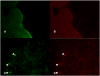
Methods: HCT-8 cells were grown in a reduced-gravity, low-shear, rotating-wall vessel (RWV) and were infected with Cryptosporidium parvum oocysts. Routine and electron microscopy (EM), immunolabeling with fluorescein-labeled Vicia villosa lectin and phycoerythrin-labeled monoclonal antibody to a 15-kD surface-membrane protein, and quantitative polymerase chain reaction (qPCR) using probes for 18s rRNA of C. parvum and HCT-8 cells were performed.
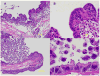
Results: The RWV allowed development of columnar epithelium-like structures. Higher magnification revealed well-developed brush borders at the apical side of the tissue. Incubation with C. parvum resulted in patchy disruption of the epithelium and, at the surface of several epithelial cells, in localized infection with the organism. EM revealed irregular stunting of microvilli, foci of indistinct tight junctions, and areas of loose paracellular spaces. qPCR showed a 1.85-log (i.e., 70-fold) progression of infection from 6 h to 48 h of incubation.
Conclusion: The HCT-8 organoid displayed morphologic changes indicative of successful and quantifiable infection with C. parvum. The HCT-8 organoid-culture system may have application in interventional in vitro studies of cryptosporidiosis.
Conflict of interest statement
All authors do not have any commercial or other association that might pose a conflict of interest.
Figures


References
-
- Adams RB, Guerrant RL, Zu S, Fang G, Roche JK. Cryptosporidium parvum infection of intestinal epithelium: morphologic and functional studies in an in vitro model. J Infect Dis. 1994 Jan;169(1):170–7. - PubMed
-
- Upton SJ, Tilley M, Brillhart DB. Comparative development of Cryptosporidium parvum (Apicomplexa) in 11 continuous host cell lines. FEMS Microbiol Lett. 1994 May 15;118(3):233–6. - PubMed
-
- Laurent F, McCole D, Eckmann L, Kagnoff MF. Pathogenesis of Cryptosporidium parvum infection. Microbes Infect. 1999 Feb;1(2):141–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

