Hypocretin and melanin-concentrating hormone in patients with Huntington disease
- PMID: 18498421
- PMCID: PMC8095609
- DOI: 10.1111/j.1750-3639.2008.00135.x
Hypocretin and melanin-concentrating hormone in patients with Huntington disease
Abstract
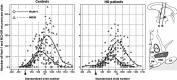
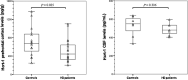
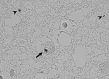
To evaluate whether hypocretin-1 (orexin-A) and melanin-concentrating hormone (MCH) neurotransmission are affected in patients with Huntington disease (HD), we immunohistochemically stained hypocretin and MCH neurons and estimated their total numbers in the lateral hypothalamus of both HD patients and matched controls. In addition, hypocretin-1 levels were determined in prefrontal cortical tissue and post-mortem ventricular cerebrospinal fluid (CSF) using a radioimmunoassay. The total number of hypocretin-1 neurons was significantly reduced by 30% in HD brains (P = 0.015), while the total number of MCH neurons was not significantly altered (P = 0.100). Levels of hypocretin-1 were 33% lower in the prefrontal cortex of the HD patients (P = 0.025), but ventricular CSF levels were similar to the control values (P = 0.306). Neuronal intranuclear and cytoplasmic inclusions of mutant huntingtin were present in all HD hypothalami, although with a variable distribution across different hypothalamic structures. We found a specific reduction in hypocretin signaling in patients with HD as MCH cell number was not significantly affected. It remains to be shown whether the moderate decrease in hypocretin neurotransmission could contribute to clinical symptoms. As the number of MCH-expressing neurons was not affected, alterations in MCH signaling are unlikely to have clinical effects in HD patients.
Figures


References
-
- Amiot C, Brischoux F, Colard C, La Roche A, Fellmann D, Risold PY (2005) Hypocretin/orexin‐containing neurons are produced in one sharp peak in the developing ventral diencephalon. Eur J Neurosci 22:531–534. - PubMed
-
- Aziz NA, Swaab DF, Pijl H, Roos RAC (2007) Hypothalamic dysfunction and neuroendocrine and metabolic alterations in Huntington disease: clinical consequences and therapeutic implications. Rev Neurosci 18:223–252. - PubMed
-
- Baumann CR, Hersberger M, Bassetti CL (2006) Hypocretin‐1 (orexin A) levels are normal in Huntington's disease. J Neurol 253:1232–1233. - PubMed
-
- Björkqvist M, Petersen A, Nielsen J, Ecker D, Mulder H, Hayden M et al (2006) Cerebrospinal fluid levels of orexin‐A are not a clinically useful biomarker for Huntington disease. Clin Genet 70:78–79. - PubMed
-
- Brundin L, Petersen A, Björkqvist M, Traskman‐Bendz L (2007) Orexin and psychiatric symptoms in suicide attempters. J Affect Disord 100:259–263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

