The taste of sugars
- PMID: 18499254
- PMCID: PMC2447812
- DOI: 10.1016/j.neubiorev.2008.04.002
The taste of sugars
Abstract
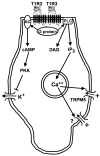
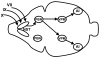
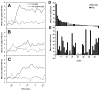
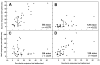
Sugars evoke a distinctive perceptual quality ("sweetness" in humans) and are generally highly preferred. The neural basis for these phenomena is reviewed for rodents, in which detailed electrophysiological measurements have been made. A receptor has been identified that binds sweeteners and activates G-protein-mediated signaling in taste receptor cells, which leads to changes in neural firing rates in the brain, where perceptions of taste quality, intensity, and palatability are generated. Most cells in gustatory nuclei are broadly tuned, so quality perception presumably arises from patterns of activity across neural populations. However, some manipulations affect only the most sugar-oriented cells, making it useful to consider them as a distinct neural subtype. Quality perception may also arise partly due to temporal patterns of activity to sugars, especially within sugar-oriented cells that give large but delayed responses. Non-specific gustatory neurons that are excited by both sugars and unpalatable stimuli project to ventral forebrain areas, where neural responses provide a closer match with behavioral preferences. This transition likely involves opposing excitatory and inhibitory influences by different subgroups of gustatory cells. Sweeteners are generally preferred over water, but the strength of this preference can vary across time or between individuals, and higher preferences for sugars are often associated with larger taste-evoked responses.
Figures

References
-
- Ackerman SH, Albert M, Shindledecker RD, Gayle C, Smith GP. Intake of different concentrations of sucrose and corn oil in preweanling rats. Am J Physiol. 1992;262:R624–7. - PubMed
-
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100(6):693–702. - PubMed
-
- Agmo A, Marroquin E. Role of gustatory and postingestive actions of sweeteners in the generation of positive affect as evaluated by place preference conditioning. Appetite. 1997;29(3):269–89. - PubMed
-
- Aleksanyan ZA, Buresova O, Bures J. Modification of unit responses to gustatory stimuli by conditioned taste aversion in rats. Physiol Behav. 1976;17(2):173–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

