Biodiversity of sphingoid bases ("sphingosines") and related amino alcohols
- PMID: 18499644
- PMCID: PMC2444003
- DOI: 10.1194/jlr.R800012-JLR200
Biodiversity of sphingoid bases ("sphingosines") and related amino alcohols
Abstract
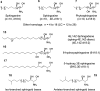
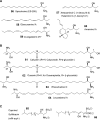
"Sphingosin" was first described by J. L. W. Thudichum in 1884 and structurally characterized as 2S,3R,4E-2-aminooctadec-4-ene-1,3-diol in 1947 by Herb Carter, who also proposed the designation of "lipides derived from sphingosine as sphingolipides." This category of amino alcohols is now known to encompass hundreds of compounds that are referred to as sphingoid bases and sphingoid base-like compounds, which vary in chain length, number, position, and stereochemistry of double bonds, hydroxyl groups, and other functionalities. Some have especially intriguing features, such as the tail-to-tail combination of two sphingoid bases in the alpha,omega-sphingoids produced by sponges. Most of these compounds participate in cell structure and regulation, and some (such as the fumonisins) disrupt normal sphingolipid metabolism and cause plant and animal disease. Many of the naturally occurring and synthetic sphingoid bases are cytotoxic for cancer cells and pathogenic microorganisms or have other potentially useful bioactivities; hence, they offer promise as pharmaceutical leads. This thematic review gives an overview of the biodiversity of the backbones of sphingolipids and the broader field of naturally occurring and synthetic sphingoid base-like compounds.
Figures







References
-
- Thudichum, J. L. W. 1884. A Treatise on the Chemical Constitution of Brain. Bailliere, Tindall, and Cox, London.
-
- Zheng W., J. Kollmeyer, H. Symolon, A. Momin, E. Munter, E. Wang, S. Kelly, J. C. Allegood, Y. Liu, Q. Peng, et al. 2006. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim. Biophys. Acta. 1758 1864–1884. - PubMed
-
- Spiegel S., and S. Milstien. 2003. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 4 397–407. - PubMed
-
- Alvarez S. E., S. Milstien, and S. Spiegel. 2007. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol. Metab. 18 300–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

