Differential dissociation of G protein heterotrimers
- PMID: 18499725
- PMCID: PMC2538816
- DOI: 10.1113/jphysiol.2008.153965
Differential dissociation of G protein heterotrimers
Abstract
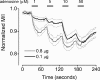
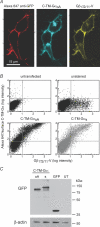
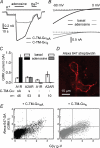
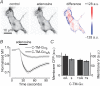
Signalling by heterotrimeric G proteins is often isoform-specific, meaning certain effectors are regulated exclusively by one family of heterotrimers. For example, in excitable cells inwardly rectifying potassium (GIRK) channels are activated by G betagamma dimers derived specifically from G(i/o) heterotrimers. Since all active heterotrimers are thought to dissociate and release free G betagamma dimers, it is unclear why these channels respond primarily to dimers released by G(i/o) heterotrimers. We reconstituted GIRK channel activation in cells where we could quantify heterotrimer expression at the plasma membrane, GIRK channel activation, and heterotrimer dissociation. We find that G(oA) heterotrimers are more effective activators of GIRK channels than G(s) heterotrimers when comparable amounts of each are available. We also find that active G(oA) heterotrimers dissociate more readily than active G(s) heterotrimers. Differential dissociation may thus provide a simple explanation for G alpha-specific activation of GIRK channels and other G betagamma-sensitive effectors.
Figures

References
-
- Akgoz M, Kalyanaraman V, Gautam N. Receptor-mediated reversible translocation of the G protein β complex from the plasma membrane to the Golgi complex. J Biol Chem. 2004;279:51541–51544. - PubMed
-
- Azpiazu I, Gautam N. A fluorescence resonance energy transfer-based sensor indicates that receptor access to a G protein is unrestricted in a living mammalian cell. J Biol Chem. 2004;279:27709–27718. - PubMed
-
- Breitwieser GE, Szabo G. Uncoupling of cardiac muscarinic and β-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985;317:538–540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

