Neuronal avalanches organize as nested theta- and beta/gamma-oscillations during development of cortical layer 2/3
- PMID: 18499802
- PMCID: PMC2396689
- DOI: 10.1073/pnas.0800537105
Neuronal avalanches organize as nested theta- and beta/gamma-oscillations during development of cortical layer 2/3
Abstract
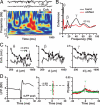
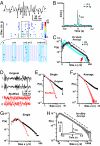
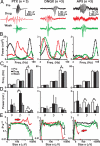
Maturation of the cerebral cortex involves the spontaneous emergence of distinct patterns of neuronal synchronization, which regulate neuronal differentiation, synapse formation, and serve as a substrate for information processing. The intrinsic activity patterns that characterize the maturation of cortical layer 2/3 are poorly understood. By using microelectrode array recordings in vivo and in vitro, we show that this development is marked by the emergence of nested - and beta/gamma-oscillations that require NMDA- and GABA(A)-mediated synaptic transmission. The oscillations organized as neuronal avalanches, i.e., they were synchronized across cortical sites forming diverse and millisecond-precise spatiotemporal patterns that distributed in sizes according to a power law with a slope of -1.5. The correspondence between nested oscillations and neuronal avalanches required activation of the dopamine D(1) receptor. We suggest that the repetitive formation of neuronal avalanches provides an intrinsic template for the selective linking of external inputs to developing superficial layers.
Conflict of interest statement
Conflict of interest statement: Provisional patent filing PCT/US2006/03 1884 “Neuronal Avalanche Assay.”
Figures


References
-
- Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444:707–712. - PubMed
-
- Khazipov R, Luhmann HJ. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 2006;29:414–418. - PubMed
-
- O'Donovan MJ. The origin of spontaneous activity in developing networks of the vertebrate nervous system. Curr Opin Neurobiol. 1999;9:94–104. - PubMed
-
- Ben-Ari Y. Developing networks play a similar melody. Trends Neurosci. 2001;24:353–360. - PubMed
-
- Ignacio MP, Kimm EJ, Kageyama GH, Yu J, Robertson RT. Postnatal migration of neurons and formation of laminae in rat cerebral cortex. Anat Embryol (Berlin) 1995;191:89–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

