Hematopoietic stem cells regulate mesenchymal stromal cell induction into osteoblasts thereby participating in the formation of the stem cell niche
- PMID: 18499897
- PMCID: PMC3513687
- DOI: 10.1634/stemcells.2008-0149
Hematopoietic stem cells regulate mesenchymal stromal cell induction into osteoblasts thereby participating in the formation of the stem cell niche
Abstract
Crosstalk between hematopoietic stem cells (HSCs) and the cells comprising the niche is critical for maintaining stem cell activities. Yet little evidence supports the concept that HSCs regulate development of the niche. Here, the ability of HSCs to directly regulate endosteal development was examined. Marrow was isolated 48 hours after "stressing" mice with a single acute bleed or from control nonstressed animals. "Stressed" and "nonstressed" HSCs were cocultured with bone marrow stromal cells to map mesenchymal fate. The data suggest that HSCs are able to guide mesenchymal differentiation toward the osteoblastic lineage under basal conditions. HSCs isolated from animals subjected to an acute stress were significantly better at inducing osteoblastic differentiation in vitro and in vivo than those from control animals. Importantly, HSC-derived bone morphogenic protein 2 (BMP-2) and BMP-6 were responsible for these activities. Furthermore, significant differences in the ability of HSCs to generate a BMP response following stress were noted in aged and in osteoporotic animals. Together these data suggest a coupling between HSC functions and bone turnover as in aging and in osteoporosis. For the first time, these results demonstrate that HSCs do not rest passively in their niche. Instead, they directly participate in bone formation and niche activities. Disclosure of potential conflicts of interest is found at the end of this article.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures
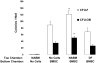
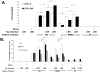




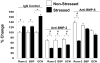


References
-
- Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439(7076):599–603. - PubMed
-
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/Angiopoietin-1 Signaling Regulates Hematopoietic Stem Cell Quiescence in the Bone Marrow Niche. Cell. 2004;118(2):149–161. - PubMed
-
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. - PubMed
-
- Nilsson SK, Johnston HM, Whitty GA, Williams B, Webb RJ, Denhardt DT, Bertoncello I, Bendall LJ, Simmons PJ, Haylock DN. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005:2004–2011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

