Telomere dysfunction and tumour suppression: the senescence connection
- PMID: 18500246
- PMCID: PMC3688269
- DOI: 10.1038/nrc2393
Telomere dysfunction and tumour suppression: the senescence connection
Abstract
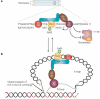
Long-lived organisms such as humans have evolved several intrinsic tumour suppressor mechanisms to combat the slew of oncogenic somatic mutations that constantly arise in proliferating stem-cell compartments. One of these anticancer barriers is the telomere, a specialized nucleoprotein complex that caps the ends of eukaryotic chromosome. Impaired telomere function activates the canonical DNA damage response pathway that engages p53 to initiate apoptosis or replicative senescence. Here, we discuss how p53-dependent senescence induced by dysfunctional telomeres may be as potent as apoptosis in suppressing tumorigenesis in vivo.
References
-
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. - PubMed
-
- Bodnar AG, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–52. - PubMed
-
- Vaziri H, Benchimol S. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr Biol. 1998;8:279–82. - PubMed
-
- Maser RS, DePinho RA. Connecting chromosomes, crisis, and cancer. Science. 2002;297:565–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous