MicroRNAs regulate ocular neovascularization
- PMID: 18500251
- PMCID: PMC3033219
- DOI: 10.1038/mt.2008.104
MicroRNAs regulate ocular neovascularization
Abstract
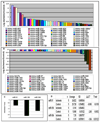
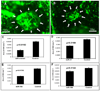
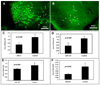
In this study, we used ischemia-induced retinal neovascularization (NV) as a model to investigate the possible role of microRNAs in a clinically important disease process. Microarray analysis demonstrated seven microRNAs (miR-106a, -146, -181, -199a, -214, -424, and -451) that were substantially increased and three microRNAs (miR-31, -150, and -184) that were substantially decreased in ischemic retina. Potential targets for the upregulated microRNAs were not identified, but bioinformatic analysis suggested target genes for the downregulated microRNAs, and these were confirmed using a luciferase reporter assay. Real-time reverse transcriptase PCR confirmed that the substantial levels of miR-31, -150, and -184 present in normal retina were significantly reduced in ischemic retina. Interestingly, constitutive levels of miR-31 and -184 are high in the cornea and lens, two avascular tissues. Intraocular injection of pre-miR-31, -150, or -184 significantly reduced ischemia-induced retinal NV, and injection of pre-miR-31 or -150 also significantly reduced choroidal NV. These data suggest that alteration of microRNA levels contributes to two types of ocular NV, and that injection or enhanced expression of microRNAs is a potential therapeutic strategy.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

