Imaging the biogenesis of individual HIV-1 virions in live cells
- PMID: 18500329
- PMCID: PMC2708942
- DOI: 10.1038/nature06998
Imaging the biogenesis of individual HIV-1 virions in live cells
Abstract
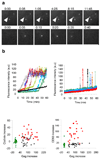
Observations of individual virions in live cells have led to the characterization of their attachment, entry and intracellular transport. However, the assembly of individual virions has never been observed in real time. Insights into this process have come primarily from biochemical analyses of populations of virions or from microscopic studies of fixed infected cells. Thus, some assembly properties, such as kinetics and location, are either unknown or controversial. Here we describe quantitatively the genesis of individual virions in real time, from initiation of assembly to budding and release. We studied fluorescently tagged derivatives of Gag, the major structural component of HIV-1-which is sufficient to drive the assembly of virus-like particles-with the use of fluorescence resonance energy transfer, fluorescence recovery after photobleaching and total-internal-reflection fluorescent microscopy in living cells. Virions appeared individually at the plasma membrane, their assembly rate accelerated as Gag protein accumulated in cells, and typically 5-6 min was required to complete the assembly of a single virion. These approaches allow a previously unobserved view of the genesis of individual virions and the determination of parameters of viral assembly that are inaccessible with conventional techniques.
Figures



References
-
- Sherer NM, et al. Visualization of retroviral replication in living cells reveals budding into multivesicular bodies. Traffic. 2003;4:785–801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

