Pharmacology of anabolic steroids
- PMID: 18500378
- PMCID: PMC2439524
- DOI: 10.1038/bjp.2008.165
Pharmacology of anabolic steroids
Abstract
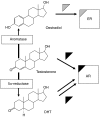
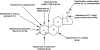
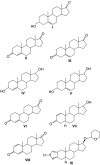
Athletes and bodybuilders have recognized for several decades that the use of anabolic steroids can promote muscle growth and strength but it is only relatively recently that these agents are being revisited for clinical purposes. Anabolic steroids are being considered for the treatment of cachexia associated with chronic disease states, and to address loss of muscle mass in the elderly, but nevertheless their efficacy still needs to be demonstrated in terms of improved physical function and quality of life. In sport, these agents are performance enhancers, this being particularly apparent in women, although there is a high risk of virilization despite the favourable myotrophic-androgenic dissociation that many xenobiotic steroids confer. Modulation of androgen receptor expression appears to be key to partial dissociation, with consideration of both intracellular steroid metabolism and the topology of the bound androgen receptor interacting with co-activators. An anticatabolic effect, by interfering with glucocorticoid receptor expression, remains an attractive hypothesis. Behavioural changes by non-genomic and genomic pathways probably help motivate training. Anabolic steroids continue to be the most common adverse finding in sport and, although apparently rare, designer steroids have been synthesized in an attempt to circumvent the dope test. Doping with anabolic steroids can result in damage to health, as recorded meticulously in the former German Democratic Republic. Even so, it is important not to exaggerate the medical risks associated with their administration for sporting or bodybuilding purposes but to emphasize to users that an attitude of personal invulnerability to their adverse effects is certainly misguided.
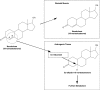
Figures

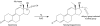

References
-
- Agarwal AK, Monder C. In vitro metabolism of 7-alpha-methyl-19-nortestosterone by rat-liver, prostate, and epididymis. Endocrinology. 1988;123:2187–2193. - PubMed
-
- Alen M, Hakkinen K. Androgenic steroid effects on serum hormones and on maximal force development in strength athletes. J Sports Med Phys Fitness. 1987;27:38–46. - PubMed
-
- Antonio J, Wilson JD, George FW. Effects of castration and androgen treatment on androgen-receptor levels in rat skeletal muscles. J Appl Physiol. 1999;87:2016–2019. - PubMed
-
- Bartsch W, Krieg M, Voigt KD. Quantification of endogenous testosterone, 5-alpha-dihydrotestosterone and 5-alpha-androstane-3-alpha, 17-beta-diol in subcellular fractions of the prostate, bulbocavernosus/levator ani muscle, skeletal muscle and heart muscle of the rat. J Steroid Biochem. 1980;13:259–264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

